In CKD Patients Stages 2-4:
7.1 The serum levels of corrected total calcium should be maintained within the normal range for the laboratory used. (EVIDENCE)
In CKD Patients with Kidney Failure (Stage 5):
7.2 Serum levels of corrected total calcium should be maintained within the normal range for the laboratory used (8.8-9.5 mg/dL [2.20-2.37 mmol/L]), and preferably toward the lower end. (OPINION)
7.3 In the event that the corrected total serum calcium level exceeds 10.2 mg/dL (2.54 mmol/L), therapies that increase serum calcium should be adjusted as follows:
7.3.a In patients taking calcium-based phosphate binders, the therapy should be discontinued and the use of non-calcium, non-metal based phosphate binders should be considered. (OPINION) See Guideline 6.
7.3.b In patients taking active vitamin D sterols, the therapy should be discontinued until the serum levels of corrected total calcium return to the target range (8.8-9.5 mg/dL [2.20-2.37 mmol/L]). (OPINION) See Guideline 9B.
7.3.c If hypercalcemia (serum levels of corrected total calcium >10.2 mg/dL [2.54 mmol/L]) persists despite discontinuation of therapy with vitamin D and/or modification of calcium-based phosphate binders, dialysis using lower dialysate calcium may be used for 3-4 weeks. (OPINION) See Guideline 10.
In CKD Patients Stages 3-5:
7.4 The total dose of elemental calcium provided by the calcium-based phosphate binders should not exceed up to 2X DRI for calcium based on age, (OPINION) and the total intake of elemental calcium (including dietary calcium) should not exceed 2,500 mg/day. (OPINION)
7.5 The serum CaXP should be maintained at <55 mg 2/dL 2 in adolescents >12 years, and <65 mg 2/dL 2 in younger children. (OPINION) This is best achieved by controlling serum levels of phosphorus within the target range. (OPINION) See Guidelines 4-5-6.
7.6 Patients whose serum levels of corrected total calcium are below the lower limit (<8.8 mg/dL [2.20 mmol/L]) should receive therapy to increase serum calcium levels:
7.6.a Therapy for hypocalcemia should include calcium salts such as calcium carbonate or calcium acetate orally, or calcium gluconate or calcium chloride parenterally (EVIDENCE), and/or oral vitamin D sterols. (EVIDENCE) See Guideline 9.
Maintenance of normal calcium balance and serum calcium levels depends on integrated regulation of calcium absorption and secretion by the intestinal tract, the excretion of calcium by the kidney, and calcium release from and deposition into bone. Parathyroid hormone increases serum calcium levels by stimulating bone resorption and kidney distal tubular calcium reabsorption in the kidney, and activating renal hydroxylation of 25(OH)D3 to 1,25(OH)2D3. Depression in serum levels of calcium by itself stimulates, through the calcium-sensing receptor (CaR) in the parathyroid gland, the secretion of preformed PTH from the parathyroid gland within seconds. Subsequently, PTH biosynthesis by the parathyroid gland increases over 24-48 hours and, if hypocalcemia persists, is followed by parathyroid gland hypertrophy and hyperplasia. Vitamin D metabolites and serum phosphorus levels also regulate PTH levels in blood. These homeostatic mechanisms are distorted in early stages of CKD and continue to deteriorate as loss of kidney function progresses.
During childhood and adolescence, total skeletal calcium increases from approximately 25 g at birth to 900 g and 1,200 g in adult females and males, respectively. Of the total body calcium, 99% is in the skeleton, 0.6% in soft tissues, and 0.1% in extracellular fluid.209 Normal values for serum total calcium concentration according to age are summarized in Table 6, Guideline 4. In adults, variations in serum levels of calcium depending on age and gender have been observed.210
Calcium in blood exists in three distinct fractions: protein-bound calcium (40%), free (formerly called ionized) calcium (48%), and calcium complexed with various anions such as phosphate, lactate, citrate, and bicarbonate (12%). Free calcium can be measured using ion-selective electrodes in most hospitals and values in adults range between 4.65-5.28 mg/dL (1.16-1.32 mmol/L).211,212 Ionized calcium should be assessed if subtle changes are expected or total calcium measurements are not adequate. Generally, measurement of ionized calcium is more expensive than total calcium measurement. For this reason, and because ionized calcium is not routinely measured, this Guideline will be based on the levels of total calcium in the blood. The latter does reflect the measured levels of ionized calcium if serum levels of protein are normal.
If serum levels of albumin are low, a correction of the measured serum levels of calcium should be made. Several formulas have been developed to correct total calcium for abnormal albumin or to calculate ionized calcium both in healthy subjects and patients with CKD, but all of them are encumbered with limitations. Also, a fall in pH of 0.1 unit will cause approximately a 0.1 mEq/L rise in the concentration of ionized calcium, since hydrogen ion displaces calcium from albumin, whereas alkalosis decreases free calcium by enhancing the binding of calcium to albumin.210
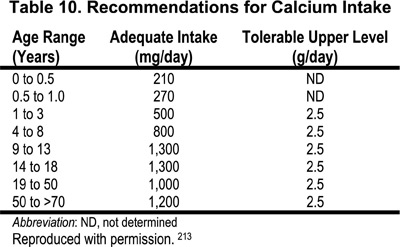
There are no biochemical measurements that reflect calcium nutritional status in subjects with normal kidney function and in patients with kidney disease. The major indirect measures of calcium nutritional adequacy are skeletal health assessed by risk of fractures, bone mass measurements, and desirable rates of calcium retention in bone. Based on these surrogate markers, the Dietary Reference Intake (DRI) Committee213 recommended the term “adequate intakes” (AIs) of calcium. This represents an approximation of the calcium intake that, in the judgment of the DRI Committee, is sufficient to maintain calcium nutriture based on observed or experimentally determined estimates of average calcium intake by groups of healthy people. The recommended dietary allowance (RDA), the term used for average daily dietary intake level that is sufficient to meet the nutritional requirements of 97%-98% of all healthy individuals in a life-stage and gender group, could not be established. At the same time, the tolerable upper level for calcium intake was established; this represents the maximal intake of calcium that is likely to pose no risks of adverse effects in healthy individuals. The examples of adequate intake and upper intake levels of calcium in various age groups of healthy subjects are presented in Table 10.
The total daily intake of elemental calcium in CKD patients should not exceed 2,500 mg per day, including both dietary sources and calcium from phosphate binders. Table 11 provides the calcium content of various commercially available calcium-based binders.
Adequate dietary intake of calcium in patients with different stages of CKD is more difficult to estimate than in healthy subjects, when one takes into consideration the changes in calcium, phosphorus, vitamin D, PTH, and bone metabolism that occur in CKD. Ideal dietary calcium intake should provide enough calcium to maintain calcium balance as close as possible to that of the age- and gender-matched healthy population. Calcium balance (intake minus the sum of all losses) in the healthy population is positive throughout childhood and adolescence. Calcium accrual is maximal during adolescence (+200 mg to +300 mg/day).213 Additionally, in CKD patients, the fraction of intestinal calcium absorption in the duodenum and jejunum is reduced141 because this process is vitamin D-dependent,214 and CKD patients have reduced blood levels of 1,25(OH)2D.215 However, passive intestinal calcium absorption, which is gradient-dependent, can be augmented by increasing calcium intake.214
Patients with CKD who are treated with metabolites of vitamin D or calcium supplementation are particularly prone to develop hypercalcemia. This complication occurs especially in those with low-turnover bone disease. The clinical presentation of hypercalcemia varies from a mild, asymptomatic, biochemical abnormality detected during routine screening to a life-threatening emergency.216,217
Hypercalcemia, together with hyperphosphatemia, or individually, can be responsible for increased blood CaXP. Since serum phosphorus levels in patients with CKD are usually increased by a higher factor compared to calcium, the relative importance of serum phosphorus levels in generating higher CaXP (expressed in mg2/dL2) is greater than the serum calcium levels. Still, the serum calcium levels could be critical218 if the serum phosphorus levels are very high, which is indeed the case in patients with Stage 5 CKD.
In the presence of high CaXP in blood, soft-tissue calcification is likely but not always associated with high CaXP, since many factors are involved in the genesis of soft-tissue calcification (see Table 6, Guideline 4).
Adequate dietary calcium intake during childhood is necessary for the development of optimal peak bone mass.219 Infants on breast milk or standard formulas should meet the DRI with the consumption of adequate volumes of breast milk/formula. For younger children (2-8 years of age), reaching the recommended levels of adequate intake of calcium is more feasible than in the 9- to 18-year-old range due to their greater acceptance of high-calcium foods in the diet. The largest source of dietary calcium for most persons is milk and other dairy products.220 Mean intakes in the 9- to 18-year-old age group are between 700-1,000 mg/day, with values at the higher end of this range occurring in males.219
An intake of 100% of the DRI for calcium is a reasonable starting point for children with CKD Stages 1-4. The challenge is to ensure that it is achieved. Infants with CKD may not get adequate amounts of calcium if breast milk is contraindicated, if low-electrolyte infant formulas are required, or if fluids are restricted. In these situations, calcium supplementation may be required. Since many high-phosphorus foods are also high in calcium, restricting dietary phosphorus will inadvertently restrict dietary calcium as well. Therefore, it is beneficial to avoid over-restriction of phosphorus or the premature initiation of a phosphorus-restricted diet in the early stages of CKD, in order to maximize dietary calcium intake.
If the patient's serum phosphorus and dietary phosphorus intake is elevated, the necessary dietary calcium intake can be accomplished with the incorporation of low-phosphorus, high-calcium foods220 (Table 12), calcium-fortified foods, and supplementation with oral calcium211-223 (Table 13). However, one should note that the food items in Table 12 characteristically do not make up a substantial part of a child's diet and may, in fact, be contraindicated as kidney function worsens and dietary potassium restriction becomes necessary.
The bioavailability of calcium from vegetables is generally high. An exception is spinach, which is high in oxalate, making the accompanying calcium virtually unavailable. Some high-phytate foods, such as bran cereal, also may have poor bioavailability of calcium.224-226
Several products have been introduced that are fortified with calcium.220 These products range from juices to breakfast foods, and it is probable that additional products will soon become available. Limited studies of the bioavailability of calcium when added to these products suggest that it is at least comparable to that of milk.222 Calcium-fortified products may be considered a practical approach to increasing the calcium intake in children/adolescents with CKD.
As noted above, supplementation should be considered if the dietary intake alone does not meet or exceed the DRI, which is often the case with progression of the kidney function through Stages 2-4. Calcium supplementation, whether a combination of calcium and gluconate (9% elemental calcium), lactate (13% elemental calcium), acetate (25% elemental calcium), or carbonate (40% elemental calcium), is well tolerated and is not toxic if used at dosages that do not exceed the DRI. Calcium carbonate—in particular—is inexpensive, tasteless, and relatively well tolerated by children of all ages with impaired kidney function.
In contrast, calcium chloride should be avoided as a supplement in uremic patients due to the possible development of metabolic acidosis. Calcium citrate should not be given to patients receiving aluminum salts since citrate augments aluminum absorption, increases the body burden of aluminum, and increases the risk of aluminum toxicity.203
When calcium salts are prescribed for the purpose of binding phosphorus in the intestine, they are more effective if given with meals. On the other hand, to ensure the optimal absorption of calcium when it is used as a supplement, it should be taken between meals.107,141,223
It is noteworthy that the dietary calcium intake of children and adolescents on dialysis who consume a phosphorus-restricted diet generally has a serious calcium deficit; typically, the estimated dietary calcium intake is <500 mg per day. Calcium-containing phosphate binders become the primary source of elemental calcium in the diet. At the same time, limiting the calcium intake from binders and dialysate solutions may be necessary in order to prevent soft-tissue calcifications as a potential consequence of long-term positive calcium balance. One must note, however, that it is impossible to accurately assess the actual absorption of calcium derived from binders, which is in large part dependent upon the kind and amount of food present in the stomach with the binder.
It is important that patients with CKD have normal serum levels of corrected total calcium, since chronic lower levels of calcium cause 2° HPT, have adverse effects on bone mineralization, and may be associated with increased mortality. Therefore, hypocalcemia should be treated. Also, adequate calcium intake in CKD patients is needed to prevent negative calcium balance. Since dietary intake of calcium in CKD patients is restricted, calcium supplementation may be required. At the same time, high calcium intake should be avoided since patients with CKD may encounter difficulties in buffering increased calcium loads, and such difficulty may result in hypercalcemia and/or soft-tissue calcification. Indeed, hypercalcemia is a frequent occurrence during therapy with calcium-based phosphate binders and/or active vitamin D sterols. Spontaneous hypercalcemia also occurs in CKD patients.
It is accepted that total calcium levels need to be adjusted for the level of albumin to better reflect the ionized calcium.210 The Evidence Report of these Guidelines cites two major studies that evaluated various formulas for correction of total calcium for albumin in 82 hemodialysis and 34 continuous ambulatory peritoneal dialysis (CAPD) patients.227,228 One of these studies used preferable statistical methods and also employed strict control of blood drawing and handling.227 Albumin was assayed by an automated bromocresol green method (BCG), total calcium by arenazo III binding, and ionized calcium by ion-selective electrode. Therefore, the equation derived from this study most closely approximates corrected total calcium in patients with CKD with an interclass correlation value of 0.84:
Corrected calcium (mg/dL) = Total calcium (mg/dL) + 0.0704 × [34 − Serum albumin (g/L)]
The use of different methods for measuring either albumin or calcium may yield different correlations from the one derived from this study. For the routine clinical interpretation of serum calcium needed for appropriate care of patients with kidney diseases, a simple formula for adjusting total serum calcium concentration for changes in serum albumin concentration can be used by clinicians.210 This formula yields similar results to that described above:
Corrected total calcium (mg/dL) = Total calcium (mg/dL) + 0.8 × [4 − Serum albumin (g/dL)]
Patients with GFR <60 mL/min/1.73 m2 (Stage 3 CKD) usually, but not invariably, show a detectable decrease in the blood levels of total and ionized calcium.229,230 The serum calcium levels decrease further as kidney function deteriorates. In advanced stages of CKD, the fraction of total calcium bound to complexes is increased231; thus, free (ionized) calcium levels are decreased despite normal total serum calcium levels. Acidosis, on the other hand, may increase the serum levels of free calcium. With initiation of regular hemodialysis, the levels of serum total calcium usually normalize.
Hypocalcemia as a risk factor for outcomes (such as increased mortality, incidence of fractures and bone disease, and quality of life) was not adequately addressed in reported clinical studies. A few studies of adults published in the early 1970s suggest that hypocalcemia may have detrimental consequences for patients with CKD.142,146,147,232-234 In one cohort study, 433 patients beginning dialysis therapy were followed prospectively for an average of 41 months.235 In 281 of the patients, the level of total calcium was <8.8 mg/dL. After adjusting for comorbid conditions, serum albumin and blood hemoglobin, chronic hypocalcemia was associated with increased mortality (P <0.006). This association was similar among patients treated with hemodialysis or peritoneal dialysis. Covariant analysis showed that hypocalcemia in these patients was associated with de novo and recurrent cardiac ischemic heart disease and congestive heart failure.
A positive relationship has been found between serum calcium level, mineralization surface, and osteoid surface.147 A statistically significant relationship between the serum calcium level and the percentage of metacarpal cortical/total bone area was found by X-ray.146 However, this was not the case when the cortical area of bone in the patients was calculated as a percentage of cortical area of bone in subjects with normal kidney function.142 Serum levels of total alkaline phosphatase activity, used as a marker of the severity of 2° HPT in patients with CKD, did not correlate with the serum levels of calcium.142,146,147,232-234 Despite a moderate significant inverse correlation between serum calcium levels and serum PTH levels,232,233 it was not possible to calculate the relative risk for development of 2° HPT for particular levels of serum calcium. Some of the more recent studies236 did not find a relationship between elevated serum levels of PTH observed in CKD patients with different levels of GFR and the levels of serum calcium, which were within the normal range independent of the stage of kidney disease.
Taken together, the results of the Evidence Report for this Guideline indicate that hypocalcemia is a risk factor for bone disease and for development of 2° HPT and/or increased risk of mortality. Thus, the detection of true hypocalcemia and its appropriate treatment is important for management of patients with CKD.
There are no data suggesting that transient mild hypercalcemia has detrimental effects on morbidity in patients with CKD. In one study, there was no evidence that isolated hypercalcemia is associated with increased morbidity in the hemodialysis population.85 Hypercalcemia poses a risk for CKD patients as it increases the CaXP in blood. Severe hypercalcemia with clinical symptoms must be treated appropriately.
Net calcium absorption is reduced in CRF as a consequence of both decreased calcium intake and decreased fraction of calcium absorbed by the intestine. The fraction of intestinal absorption of calcium is decreased early in the course of kidney disease. This is observed in Stage 3 CKD and worsens as CKD progresses.107,141,237-239 Initiation of dialysis does not improve calcium absorption.107,238,239 It is common to observe significant variability in intestinal calcium absorption within a group of patients with the same degree of kidney dysfunction,107,141,237-239 and, therefore, population studies may not be adequate to address the status of intestinal calcium absorption in individual patients.
Dietary calcium intake is low in patients with CKD. Intake of calcium in adults with advanced CKD ranged between 300-700 mg/day107,240; in those treated with hemodialysis, calcium intake averaged 549 mg/day241; and it was 80% of the recommended daily allowance in children with GFR between 20-75 mL/min/1.73 m2.242 When dietary calcium intake was <20 mg/kg/day, patients with CKD had negative net intestinal calcium balance, but neutral calcium balance was achievable with calcium intake around 30 mg/kg/day.243
There are no data on calcium retention as a function of increased long-term calcium intake in patients with CKD. In data calculated for healthy adolescents, young adults, and adult men, calcium retention reached a plateau despite an increase in calcium intake from 1,000-2,500 mg/day.214 Thus, we are poorly equipped to establish values for adequate intake of calcium in patients with kidney disease. The opinion of the Work Group is that an intake of 2X age-specific DRI (maximum 2,500 mg/day) of calcium (dietary and supplements) is appropriate for CKD patients.
While this recommendation of the Work Group is not based on evidence provided in the Evidence Report, there are data from different studies identifying the requirement of calcium for various components of calcium balance (intestinal calcium absorption and calcium secretion) and calcium losses (urinary, fecal, and sweat) in CKD patients (Table 14). These data show that the requirement of daily calcium intake in Stage 3 CKD is 1.5X to 2X age-specific DRI (maximum 2,500 mg/day) and in Stages 4 and 5 CKD (patients not on dialysis), it is 1.5-1.8 g/day. The Work Group's recommendation of total daily calcium intake of 2X age-specific DRI (maximum 2,500 mg/day) is in agreement with these data.
Furthermore, in dialysis patients, calcium supplementation of 3.0 g/day in addition to the 400-500 mg in dietary calcium resulted in hypercalcemia in up to 36% of patients.244 Other studies show lower, but still significant, incidences of hypercalcemia during high calcium intake.171,245 This clearly suggests that there is a tolerable upper intake level for patients with CKD and, therefore, higher daily calcium intake (>2X age-specific DRI [maximum 2500 mg/day]) should be avoided.
The effectiveness of different calcium salts used for calcium supplementation was partially addressed by four studies.21,205,246,247 Only one of these studies247 directly compared the efficacy of two different calcium salts (calcium carbonate versus calcium citrate). However, this study followed the patients for only 3 hours after administration of the calcium supplements, and therefore the results represent only short-term effects. The other three studies compared the use of calcium carbonate to placebo or no calcium supplement. Because of the different study conditions and patient populations, and because these studies did not directly address the question being asked, it was not useful to conduct a meta-analysis. Therefore, the recommendation for the use of calcium carbonate for calcium supplementation in this Guideline is opinion-based and endorsed by the Work Group.
Similarly, the four studies cited above did not provide information that could be utilized to ascertain whether giving the calcium salts before, during, or after meals is more effective. Further, the data are not helpful in deciding whether it is better to give the calcium salts in one dose per day or divided into multiple doses.
The question as to when to initiate calcium supplementation during the course of CKD is not answered by the available data in the literature. Certainly, in the presence of overt hypocalcemia, calcium supplementation is indicated. However, determining when to initiate calcium therapy in patients with CKD involves a consideration of multi-dimensional biological parameters on the part of the clinician. It seems, however, that calcium supplementation should be considered in CKD patients when serum levels of PTH begin to rise.
In adults, an association was observed between CaXP and the risk of death in a random sample of the U.S. population of 2,669 patients treated for at least 1 year with hemodialysis from 1990-1993.85 Patients with CaXP >72 (20% of all patients) had a 34% higher relative risk of death compared to patients with CaXP in the range of 42-52.85 The increased risk was observed in proportion to the elevation of CaXP; indeed, for every increase of 10 in CaXP, there was an 11% increase in relative risk of death.
The Evidence Report cites four studies that address the issue of CaXP as a risk for soft-tissue calcification. One prospective, uncontrolled study of 137 patients showed that 35 patients ages 55 to 64, with poorly controlled CaXP (above 60) had increased aortic calcification index (ACI = 26.1) as compared to 20 patients of the same age with well-controlled CaXP ‹60 (ACI = 17.7).79 Another prospective, controlled study using stepwise discrimination analysis showed significantly higher risk for mitral annular calcification in hemodialysis patients with CaXP of 63 ±13 compared to those with CaXP of 56 ±13.248 An additional study showed that, in young adults on hemodialysis who had CaXP of 65 ±10.6, coronary artery calcification was significantly higher than in those with CaXP of 56 ±12.7.80 One retrospective, controlled study in CAPD patients showed no significant differences in CaXP in 17 patients with mitral annular calcification as compared to 118 patients without this abnormality.249 Despite the fact that these studies were not controlled for potential confounding variables and are encumbered with selection bias, it seems reasonable to conclude that high levels of CaXP can pose a risk of vascular calcification.
The level of CaXP in CKD patients at which risk for calcification is very low or unlikely to occur, has been debated over the last 40 years, but no strong evidence is available to answer this question. As discussed above, CaXP levels are most likely a risk for calcification, but assessing calcification risk does not involve arriving at “yes” or “no” answers. The theory is that calcification risk increases as CaXP increases; however, evidence on this relationship is scant and is presented below.
Studies in adults and children80,93,248-253 examined the calcium phosphorus product as a risk for extraskeletal calcification. None examined risk for future calcification. All were cross-sectional studies. Four were retrospective249,251-253 and four prospective.80,93,248-250 They used different methods (radiography, scintigraphy, CT, echocardiography) for detection of calcification and examined different organs for calcification (e.g., soft tissue, mitral and aortic valve, aorta, lung). Two studies249,252 provided enough information to calculate risk ratios for CaXP for inducing soft-tissue calcification. One study249 included 135 Stage 5 CKD predialysis patients and 76 patients on CAPD, and the other252 reported on 47 patients for more than 2 years on CAPD. This limited information suggests that CaXP may be a useful indicator of calcification in patients with Stage 5 CKD, as no trend for risk was seen.249 Data on patients treated with CAPD for 2 years showed that the risk for mitral calcification increased as CaXP increased.252 In contrast, in patients treated with CAPD for 1 year, there was no relationship between the risk for calcification and the levels of CaXP.249 The confidence intervals in this small study are very wide, and thus firm conclusions cannot be reached. Neither of these studies examined whether CaXP can be used as a predictor of future calcification.
Two case-controlled studies indicated that there were significant differences in CaXP between patients with and without aortic valve calcification and mitral annular calcification248,251 and normal and abnormal visceral uptake of 99Tc-PP or 99Tc-MDP.250
The incidence of visceral calcification in a selected dialysis population was high when mean CaXP exceeded 68 and low when mean CaXP was 51.250 Frequent incidence of visceral calcification248 and mitral valve calcification251was reported when CaXP exceeded 60 and calcification was unlikely when mean CaXP was around 50.250,251 It must be noted that a significant number of patients did not develop extraskeletal calcification despite a high CaXP.80,250,251
Thus, the available evidence is limited, but convincing, that primary outcome (increased death rate) and secondary outcome (extraskeletal calcification) are related to CaXP. If this value exceeds 55, there is increased risk for development of calcification and possibly increased risk for lower patient survival. Thus, the goal level of CaXP should be below 55 in adolescents, and below 65 in infants and children, due to the normal higher serum phosphorus level in the latter groups.
There are no evidence-based studies in adults or children to define an upper limit of calcium intake to help prevent metastatic calcifications. There are no prospective studies that address imaging modalities to detect extraskeletal calcification in children. There are no prospective studies that address the risk factors for vascular calcifications in childhood years. There are no studies in children with vascular calcifications to understand how the lesions may be best treated to promote regression.
Calcium needs of infants, children, and adolescents in Stages 1-5 of CKD should be determined by prospective, longitudinal studies. Factors that regulate the percentage of calcium absorption in the gastrointestinal tract with the use of calcium-containing binders in patients of different ages with CKD and receiving either peritoneal dialysis or hemodialysis should be determined.
Longitudinal studies of patient morbidity (e.g., growth, vascular calcifications) as a function of calcium intake should be determined.