THE WORK GROUP expanded on selected clinical topics that were not included in the scope of the review of evidence, but which nonetheless are relevant to the implementation of a clinical action plan for patients with chronic kidney disease. The clinical approach outlined below is based on guidelines contained within this report; the reader is cautioned that many of the recommendations in this section have not been adequately studied and therefore represent the opinion of members of the Work Group.
Assessment of Risk
All individuals should be evaluated during health encounters to determine whether they are at increased risk of having or of developing chronic kidney disease. Guideline 3 lists risk factors for susceptibility to and initiation of chronic kidney disease (“CKD risk factors”). Ascertainment of risk factors through assessment of sociodemographic characteristics, review of past medical history and family history, and measurement of blood pressure would enable the clinician to determine whether a patient is at increased risk. Patients who are found to be at increased risk should be evaluated further.
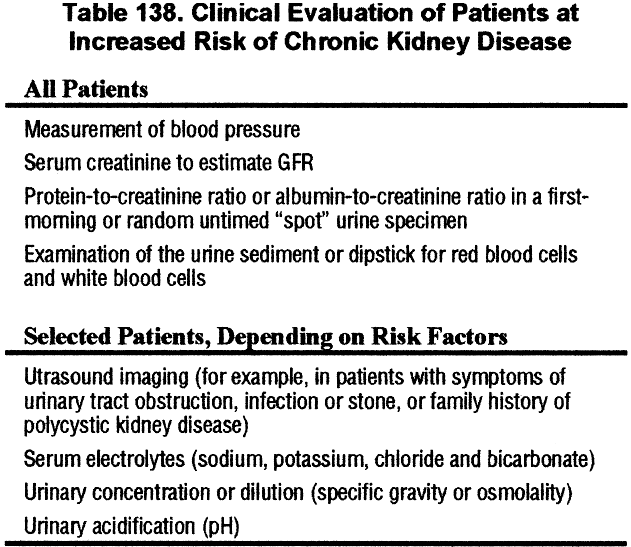
Clinical Evaluation of Patients at Increased Risk
Clinical evaluation of patients at increased risk of chronic kidney disease includes assessment of markers of kidney damage, estimated GFR, and blood pressure (Table 138).
Unfortunately, these markers do not detect all types of chronic kidney damage. Thus, it may be difficult to detect the onset of some types of chronic kidney disease until GFR is decreased, for example, hypertensive nephrosclerosis and noninflammatory tubulointerstitial diseases.
Testing for Proteinuria
The algorithms recommended by NKF PARADE distinguish between individuals at increased for chronic kidney disease versus asymptomatic, healthy individuals. These algorithms have been modified by the Work Group with input from members of the PARADE Work Group (Fig 57).
Figure 57 |
|
Evaluation of proteinuria in patients not known to have kidney disease. Modified from NKF PARADE. NKF PARADE does not use gender-specific definitions for abnormal albumin-to-creatinine ratio. Modified with permission.6 |
|
The algorithm for adults and children at increased risk (right side) begins with testing of a random “spot” urine sample with an albumin-specific dipstick. Alternatively, testing could begin with a spot urine sample for albumin-to-creatinine ratio. The algorithm for asymptomatic healthy individuals (left side) does not require testing specifically for albumin. This algorithim is useful for children without diabetes, in whom universal screening is recommended. Universal screening is not currently recommended for adults.
Clinical Presentation
Table 139 shows the relationship between stages of chronic kidney disease and clinical presentations. During the stage “At Increased Risk” and Stage 1 (Kidney Damage), specific diseases are associated with specific risk factors and are manifested by specific clinical presentations, although markers for each diagnosis have not been discovered. During Stages 2 through 4 (Decreased GFR) and Stage 5 (Kidney Failure), different diseases may have similar clinical presentations, although markers of kidney damage may persist and provide clues to diagnosis.
Simplified Classification of Chronic Kidney Disease
Diseases of the kidney are classified according to etiology and pathology. A simplified classification is given in Table 140.
Definitive diagnosis often requires a biopsy of the kidney, which is associated with a risk, albeit usually small, of serious complications. Therefore, kidney biopsy is usually reserved for selected patients in whom a definitive diagnosis can be made only by biopsy and in whom a definitive diagnosis would result in a change in either treatment or prognosis. In most patients, diagnosis is assigned based on recognition of well-defined clinical presentations and causal factors based on clinical evaluation.
Clinical Evaluation
Chronic kidney disease is usually silent. Therefore, clinical assessment relies heavily on laboratory evaluation and diagnostic imaging. Nonetheless, a careful history will often reveal clues to the correct diagnosis (Table 141).
Blood pressure measurement is essential, but other elements of the physical examination are usually not helpful, except to assess comorbid conditions and complications of decreased GFR. A number of drugs can be associated with chronic kidney damage, so a thorough review of the medication list (including prescribed medications, over-the-counter medications, “nontraditional” medications, vitamins and supplements, herbs, and drugs of abuse) is vital. Moreover, medications will require adjustment in dosage or discontinuation based on the level of GFR.
Laboratory Evaluation
Laboratory evaluation in all patients with chronic kidney disease should be performed (Table 142).
Guideline 6 provides a guide to interpretation of proteinuria and urine sediment abnormalities and findings on imaging studies as markers of kidney damage and a definition of clinical presentations.
Based on these measurements, the clinician can usually define the clinical presentation, thereby narrowing the differential diagnosis and guiding further diagnostic evaluation, decisions about kidney biopsy, and, often, decisions about treatment and prognosis with no need for kidney biopsy.
Relationships Among Type and Stage of Kidney Disease and Clinical Presentations
Tables 143, 144, and 145 show the relationships between stage of kidney disease and clinical features for diabetic kidney disease, nondiabetic kidney diseases, and diseases in the kidney transplant.
Utility of Proteinuria in Diagnosis, Prognosis, and Treatment
Proteinuria is a key finding in the differential diagnosis of chronic kidney disease. Proteinuria is a marker of damage in diabetic kidney disease (Table 143), in glomerular diseases occurring in the native kidney (Table 144), and in transplant glomerular disease and recurrent glomerular disease in the transplant (Table 145). In these diseases, the magnitude of proteinuria is usually >1,000 mg/g (except in early diabetic kidney disease), and may approach nephrotic range (spot urine protein-to-creatinine ratio >3,000 mg/g). On the other hand, proteinuria is usually mild or absent in vascular diseases, tubulointerstitial diseases, and cystic diseases in the native kidney and in rejection and drug toxicity due to cyclosporine or tacrolimus in the transplant.
Proteinuria is also a key prognostic finding. It is well-known that nephrotic range proteinuria is associated with a wide range of complications, including hypoalbuminemia, edema, hyperlipidemia, and hypercoagulable state; faster progression of kidney disease; and premature cardiovascular disease. However, it is now known that elevated urine protein excretion below the nephrotic range is also associated with faster progression of kidney disease and development of cardiovascular disease. Furthermore, the reduction in proteinuria is correlated with a subsequent slower loss of kidney function.
Finally, proteinuria is also a guide to therapy. The benefit of antihypertensive therapy, especially with angiotensin-converting enzyme inhibitors, to slow the progression of kidney disease is greater in patients with higher levels of proteinuria compared to patients with lower levels of proteinuria.
In summary, proteinuria is not only a marker of kidney damage, it is also a guide to the differential diagnosis, prognosis, and therapy of chronic kidney disease.
Guideline 13 reviews estimating decline in GFR and treatments to slow the GFR decline in adults. In general, GFR should be estimated from serum creatinine at least yearly in patients with chronic kidney disease and more often in patients with:
Treatments to slow the progression of chronic kidney disease in adults in are shown in Table 146.
Guideline 15 concludes that patients with chronic kidney disease have a high risk of adverse outcomes of cardiovascular disease and should be considered in the “highest-risk group” for cardiovascular disease risk reduction. However, few patients with chronic kidney disease have been included in population-based epidemiologic studies of cardiovascular disease or long-term randomized clinical trials. The NKF Task Force on Cardiovascular Disease in Chronic Renal Disease recommended risk factor reduction for “traditional” risk factors based largely on extrapolation from the general population and evidence of safety and efficacy of interventions on risk factor levels in chronic kidney disease. It was the opinion of the CVD Task Force and the CKD Work Group that extrapolation from the general population to patients with chronic kidney disease is most appropriate for patients with higher levels of GFR (Stages 1 through 4) and less (but possibly still) appropriate for patients with kidney failure (Stage 5). A partial list of “traditional” cardiovascular disease risk factors and risk factor reduction strategies that are potentially safe and effective for patients with chronic kidney disease is shown in Table 147.
Guidelines 7 through 12 show the associations between level of GFR and complications of chronic kidney disease in adults. Many complications begin to occur at GFR <60 mL/min/ 1.73 m2.
Table 148 lists additional clinical evaluations (in addition to the ones listed in Tables 105 and 142) that should be performed in adults with GFR <60 mL/min/1.73 m2.
Guideline 1 defines a decrease in GFR of 60 to 89 mL/min/1.73 m2 as chronic kidney disease only if accompanied by a marker of kidney damage. GFR declines with age in normal individuals; therefore, it can be difficult to distinguish age-related decrease in GFR from chronic kidney disease in the elderly. Other causes of chronically decreased GFR in normal individuals without chronic kidney disease include a habitually low protein intake and unilateral nephrectomy.
Data from NHANES III suggest that almost 75% of individuals ≥ 70 years old may have GFR <90 mL/min/1.73 m2, and almost 25% may have GFR <60 mL/min/1.73 m2. The fraction of elderly individuals with decreased GFR who truly have chronic kidney disease has not been systematically studied. Moreover, the health outcomes of decreased GFR in the elderly, with or without chronic kidney disease, are also not known.
Clinical evaluation of elderly individuals with GFR of 60 to 89 mL/min/1.73 m2 should include an assessment for chronic kidney disease (Table 149).
Additional items for clinical evaluation of individuals with GFR <60 mL/min/1.73 m2 are listed in Table 148.
It is the opinion of the members of the Work Group that clinical interventions for the elderly with chronic kidney disease should be based on diagnosis (as described above), severity of kidney function impairment, and stratification of risk for progression of kidney disease and cardiovascular disease. There is a spectrum of risk for adverse outcomes.
Patients with mild decreased GFR, low risk for progressive decline in GFR, and low risk for cardiovascular disease have a good prognosis and may require only adjustment of the dosage of drugs that are excreted by the kidney, monitoring of blood pressure, avoidance of drugs and procedures with risk for acute kidney failure, and life-style modifications to reduce the risk of cardiovascular disease. Consultation with a nephrologist may be necessary to establish the diagnosis and treatment of the type of kidney disease. Kidney function should be monitored at least yearly.
Patients with moderately or severely decreased GFR or risk factors for faster decline in GFR or cardiovascular disease have a worse prognosis. In addition to the interventions mentioned above, they require assessment for complications of decreased GFR and dietary and pharmacologic therapy directed at slowing the progression of kidney disease and ameliorating cardiovascular risk factor levels. Consultation and/or co-management with a kidney disease care team is advisable during Stage 3, and referral to a nephrologist in Stage 4 is recommended. Kidney function may need to be monitored four times per year or more. A multidisciplinary team approach may be necessary to implement and coordinate care.