GUIDELINE 1. DEFINITION AND STAGES OF CHRONIC KIDNEY DISEASE
Adverse outcomes of chronic kidney disease can often be prevented or delayed through early detection and treatment. Earlier stages of chronic kidney disease can be detected through routine laboratory measurements.
- The presence of chronic kidney disease should be established, based on presence of kidney damage and level of kidney function (glomerular filtration rate [GFR]), irrespective of diagnosis.
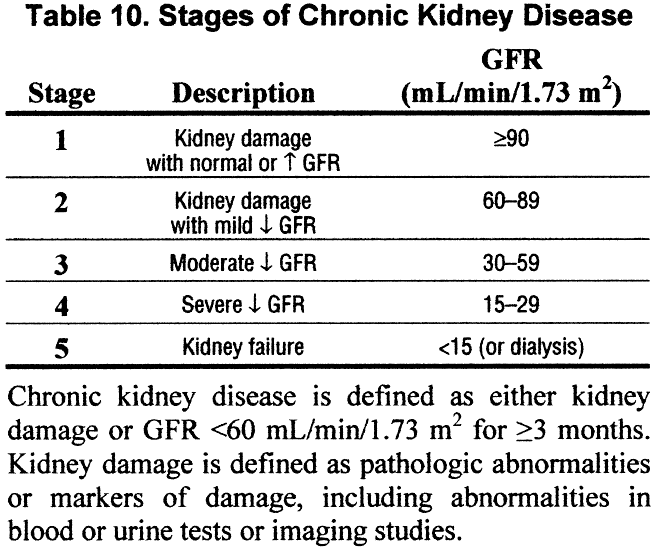
- Among patients with chronic kidney disease, the stage of disease should be assigned based on the level of kidney function, irrespective of diagnosis, according to the KDOQI CKD classification Table 10.
Chronic kidney disease is a major public health problem. Adverse outcomes of chronic kidney disease can be prevented through early detection and treatment. Earlier stages of chronic kidney disease can be detected through routine laboratory measurements.
The USRDS provides reliable nationwide data regarding the incidence, prevalence, treatment patterns, outcomes, and cost of kidney failure treated by dialysis and transplantation, the most severe stage of chronic kidney disease. This guideline provides a definition of chronic kidney disease as well as definitions and estimates of prevalence of earlier stages of kidney disease.
Chronic kidney disease is defined according to the presence or absence of kidney damage and level of kidney function—irrespective of the type of kidney disease (diagnosis). Among individuals with chronic kidney disease, the stages are defined based on the level of kidney function. Identifying the presence and stage of chronic kidney disease in an individual is not a substitute for accurate assessment of the cause of kidney disease, extent of kidney damage, level of kidney function, comorbid conditions, complications of decreased kidney function, or risks for loss of kidney function or cardiovascular disease in that patient. Defining stages of chronic kidney disease requires “categorization” of continuous measures of kidney function, and the “cut-off levels” between stages are inherently arbitrary. Nonetheless, staging of chronic kidney disease will facilitate application of clinical practice guidelines, clinical performance measures and quality improvement efforts to the evaluation, and management of chronic kidney disease.
Definition and Classification
Definition of chronic kidney disease (O). Chronic kidney disease has been defined according to the criteria listed in Table 11.
Stages of chronic kidney disease (R, O). Among individuals with chronic kidney disease, the stage is defined by the level of GFR, with higher stages representing lower GFR levels.
Table 12 illustrates the classification of individuals based on the presence or absence of markers of kidney disease and level of GFR, according to definition and staging of chronic kidney disease proposed by this guideline. In addition, it includes columns for the presence or absence of high blood pressure, because of the complex relationship of high blood pressure and chronic kidney disease.
All individuals with GFR <60 mL/min/1.73 m2 for ≥ 3 months are classified as having chronic kidney disease, irrespective of the presence or absence of kidney damage. The rationale for including these individuals is that reduction in kidney function to this level or lower represents loss of half or more of the adult level of normal kidney function, which may be associated with a number of complications (Part 6).
All individuals with kidney damage are classified as having chronic kidney disease, irrespective of the level of GFR. The rationale for including individuals with GFR ≥ 60 mL/min/1.73 m2 is that GFR may be sustained at normal or increased levels despite substantial kidney damage and that patients with kidney damage are at increased risk of the two major outcomes of chronic kidney disease: loss of kidney function and development of cardiovascular disease (Part 7).
The methods to estimate GFR and assess markers of kidney damage are not completely sensitive or specific in detecting decreased GFR and kidney damage, respectively. Thus, misclassification is possible, and clinicians should carefully consider all aspects of the patient’s clinical presentation in interpreting test results and determining evaluation and management. For the definition of chronic kidney disease, the Work Group selected cut-off levels for GFR and markers of kidney damage that maximize specificity, acknowledging potential loss of sensitivity. Clinicians should be especially careful in the evaluation of individuals with borderline abnormal results for markers of kidney disease, mild decrease in GFR (60 to 89 mL/min/1.73 m2), high blood pressure, and of other individuals at increased risk of chronic kidney disease. Risk factors for chronic kidney disease are discussed in Guideline 3.
Decreased GFR without kidney damage (R, O). Individuals with GFR 60 to 89 mL/min/1.73 m2 without kidney damage are classified as “decreased GFR.” Decreased GFR without recognized markers of kidney damage is very frequent in infants and older adults, and is usually considered to be “normal for age.” The age-related decline in GFR in adults is accompanied by pathological findings of global glomerular sclerosis and cortical atrophy. The consequences of declining GFR with age have not been carefully studied. It is interesting to speculate whether the increasing incidence of end-stage renal disease in the elderly could be due, in part, to age-associated decline in GFR.
Other causes of chronically decreased GFR without kidney damage in adults include vegetarian diets, unilateral nephrectomy, extracellular fluid volume depletion, and systemic illnesses associated with reduced kidney perfusion, such as heart failure and cirrhosis. It is not certain whether individuals with chronically decreased GFR in the range of 60 to 89 mL/min/1.73 m2 without kidney damage are at increased risk for adverse outcomes, such as toxicity from drugs excreted by the kidney or acute kidney failure. After much discussion and input from expert reviewers, the Work Group concluded that there is insufficient evidence to label individuals with GFR 60 to 89 mL/min/1.73 m2, but without markers of kidney damage, as having chronic kidney disease. In clinical practice, it may be difficult to determine whether individuals with decreased GFR have chronic kidney disease. Recommendations for a clinical approach to elderly individuals with decreased GFR is given in Part 9.
High blood pressure in chronic kidney disease and in individuals with decreased GFR without kidney disease (R). High blood pressure is not included in the definition of chronic kidney disease or its stages. However, high blood pressure is a common cause and consequence of chronic kidney disease, and as reviewed later, patients with chronic kidney disease and high blood pressure are at higher risk of loss of kidney function and development of cardiovascular disease. High blood pressure is also common in older individuals without chronic kidney disease and is associated with accelerated GFR decline with age and more marked pathological abnormalities in the kidneys. Individuals with high blood pressure should be carefully evaluated for the presence of chronic kidney disease, especially those with decreased GFR.
Prevalence of chronic kidney disease and level of kidney function in the general population (S). The prevalence of chronic kidney disease, based on the definition above, was estimated using data from NHANES III and USRDS (Fig 7 and Tables 13 and 14).
Figure 7 |
|
Prevalence
of albuminuria and high blood pressure (%) in US adults age |
|
For the analysis of NHANES III data, GFR was estimated from serum creatinine concentration using a prediction equation derived from the Modification of Diet in Renal Disease (MDRD) Study,17,18 elevated urine albumin-to-creatinine ratio was taken as a marker of chronic kidney disease, and high blood pressure was defined as blood pressure ≥ 140/90 mm Hg or taking medications for high blood pressure. These parameters were ascertained on a single occasion. A subgroup of NHANES III participants underwent repeat measurement of albuminuria. Elevated albumin-to-creatinine excretion was persistent in 61% of the subjects with albuminuria (n = 163). Therefore, these estimates of prevalence should be considered as rough approximations of the true prevalence. The rationales for these assumptions and cut-off levels are discussed in more detail below.
Kidney Damage
Definition (O) Kidney damage is defined as structural or functional abnormalities of the kidney, initially without decreased GFR, which over time can lead to decreased GFR. As described earlier, markers of kidney damage include abnormalities in the composition of the blood or urine or abnormalities in imaging tests. This section will emphasize proteinuria as a marker of kidney damage because it has been studied most thoroughly, including in NHANES III.
Proteinuria as a marker of kidney damage (R). Proteinuria is an early and sensitive marker of kidney damage in many types of chronic kidney disease. Albumin (molecular weight [MW] = 68,000 daltons) is the most abundant urine protein in most types of chronic kidney disease. Low molecular weight (LMW) globulins are the most abundant urine proteins in some types of chronic kidney disease. In this and later guidelines, the term proteinuria includes albuminuria, increased urinary excretion of other specific proteins, and increased excretion of total urine protein. On the other hand, the term albuminuria has been used only when referring to increased urinary albumin excretion. Older laboratory methods, such as the urine dipstick or acid precipitation, detect most urine proteins. Microalbuminuria refers to excretion of small but abnormal amounts of albumin, which requires recently developed, more sensitive laboratory methods that are now widely available.
Normal protein excretion (S, R). Normal mean value for urine albumin excretion in adults is approximately 10 mg/d. Albumin excretion is increased by physiological variables, such as upright posture, exercise, pregnancy, and fever. Normal mean value for urine total protein is approximately 50 mg/d. Major constituents of normal urine protein are albumin, LMW proteins filtered from the blood, and proteins derived from the urinary tract.
In practice, it is difficult to collect a timed urine specimen. As described in Guideline 5, the urinary excretion rate for albumin and total protein can be estimated from the ratio of albumin or total protein to creatinine concentration in an untimed (“spot”) urine specimen. Because protein excretion varies throughout the day, the normal ratio varies throughout the day. The ratio in a first morning specimen correlates most closely with overnight protein excretion rate, whereas the ratio in mid-morning specimens correlates most closely with 24-hour protein excretion rate. Creatinine excretion is higher in normal men than women; therefore, the values in the general population (Fig 8) and cut-off values for abnormalities in urine albumin-to-creatinine ratio are lower for men than women (Table 15).
Figure 8 |
|
Distribution
of albumin-to-creatinine ratio in US men and women, NHANES III (1988-1994), age |
|
Definition of proteinuria and albuminuria in adults (R). Table 15 shows definitions for proteinuria and albuminuria, including gender specific cut-off values for microalbuminuria and albuminuria. Cut-points for definition of abnormal urine total protein and albumin are set to maximize specificity (avoid false positives), thus, the upper limit of “normal” typically extends far above the normal mean value, resulting in low sensitivity (many false negatives).
Normal albumin excretion in children (C). Normal values for albumin excretion in children are not well established. Although increased urine albumin excretion reflects glomerular injury better than other urinary proteins in both adults and children, many pediatric nephrologists continue to monitor levels of total protein rather than albumin in patients with proteinuria. Hence, reports of normal albumin rates in children are relatively few in number, and most have been published in the past 15 years. However, a literature search of articles describing albumin excretion in children revealed one study in 1970. This original paper20 considered the best measurement of glomerular integrity to be albumin clearance factored by creatinine clearance. It concluded that the ratio of the concentration of albumin to creatinine in spot urine samples is the most accurate method for estimating albumin clearance and provides a better marker of glomerular permeability to albumin than the 24-hour albumin excretion rate. The results were expressed as mg albumin per mg creatinine, but subsequent papers have used a variety of methods to express albumin excretion, making comparisons between studies very difficult. Tables 16 and 17 give mean values and ranges for albumin excretion rate and albumin-to-creatinine ratio in children (neonates through age 20 years), and also emphasize some of the ways in which published reports have differed. Overall, the values appear similar to the values observed for adults.
Prevalence of proteinuria in adults (S). Table 18 shows the prevalence of albuminuria estimated from the albumin-to-creatinine ratio in a single spot urine collection in 14,836 adults studied in NHANES III.
Based on these results, it is estimated that approximately 20.2 million adults (11.7%) have abnormal urine albumin excretion.
Albuminuria was persistent on repeat evaluation in only 61% of individuals; hence, these prevalence estimates based on a single spot urine are likely overestimates, especially for microalbuminuria. (Appendix 2 discusses the reproducibility of data on albuminuria and microalbuminuria.)
Among adults, the prevalence of albuminuria varies by age (Table 19) and presence (Table 20) or absence (Table 21) of diabetes.
The prevalence is approximately 30% in adults with age ≥ 70 years: 26.6% with microalbuminuria and 3.7% with albuminuria. At all ages, the prevalence is higher among individuals with diabetes. Among individuals with a history of diabetes, the prevalence of microalbuminuria and albuminuria is 43.2% and 8.4%, respectively, at age ≥ 70 years. Among individuals without a history of diabetes the prevalence of microalbuminuria and albuminuria is 24.2% and 3.0%, respectively, at age ≥ 70 years.
Prevalence of proteinuria in children (C). Prevalence of proteinuria is lower in children. A compilation of studies shows that 1% to 10% of children may have proteinuria on initial screening using the urine dipstick, but that <1% have persistent proteinuria, as defined by positive results on repeated testing (Table 22).
Similarly, the prevalence of increased urine albumin excretion on initial screening varies from 1% to 10% (Table 23).
Prevalence of Stage 1 and Stage 2 chronic kidney disease (S). The proportion of adults with GFR ≥ 90 and 60–89 mL/min/1.73 m2 with albuminuria is shown in Fig 7. Among US adults with a GFR ≥ 90 mL/min/1.73 m2, 9.2% had an elevated albumin-to-creatinine ratio (including 3.3% without hypertension and 5.9% with hypertension). As shown in Table 14, this group corresponds to approximately 5.9% of all US adults, or 10.5 million people in the years 1988 to 1994. On repeat examination, 54% (n = 102) of a subsample with albuminuria had a persistently positive result. Therefore, the prevalence of persistent albuminuria would be 3.3% of US adults with GFR ≥ 90 mL/min/1.73 m2, or 5.9 million. This is the estimated prevalence of Stage 1 chronic kidney disease.
Among adults with GFR 60 to 89 mL/min/1.73 m2, the prevalence of albuminuria was 12.9%, corresponding to 4.0% of all US adults, or 7.1 million people. On repeat examination, 73% of a subsample with albuminuria (n = 44) had a persistently positive test. Therefore, the prevalence of persistent albuminuria would be 3.0% of US adults with GFR 60-84 mL/min/1.73 m2, or 5.3 million. This is the estimated prevalence of Stage 2 chronic kidney disease.
Note that persistent albuminuria is not the only marker of kidney damage. NHANES III did not ascertain other markers of kidney damage, such as abnormalities of the urine sediment and abnormal imaging tests; thus, any estimate based on NHANES III data is likely to underestimate the true prevalence of chronic kidney damage.
Decreased GFR
GFR as an index of kidney function (R). The level of GFR is accepted as the best measure of overall kidney function in health and disease. In principle, the level of GFR is the product of the number of nephrons and the single nephron GFR. Therefore, GFR can be affected by chronic kidney disease, which reduces the number of nephrons, or by hemodynamic factors that affect single nephron GFR. In chronic kidney disease, as in normal individuals, GFR is modulated by hemodynamic factors.
Normal range and variability of GFR (S, R). The normal level of GFR varies according to age, gender, and body size. It is conventional to adjust GFR to “standard” body size (surface area of 1.73 m2). Among normal adults, the inter-individual coefficient of variation (standard deviation divided by the mean) of GFR (adjusted for body surface area) within the normal population is approximately 15% to 20%.67 The normal mean (standard deviation) GFR in young adults is approximately 120 to 130 (20 to 25) mL/min/1.73 m2. Children reach adult values for mean GFR by approximately age 2 years (Table 24)68,69.
Figure 9 and Table 25 show the range of GFR in adults according to age, derived from normal men using inulin clearance.72
Figure 9 |
|
Fig 9. GFR versus age. Estimated GFR percentiles for the US population using NHANES III serum creatinine, age, sex, and race data (see Part 10, Appendix 2) by age compared to a regression of inulin clearance measurement of GFR on age among 70 healthy male participants. (Data abstracted from Davies and Shock.72) |
|
Normal values in women are assumed to be 8% lower at all ages.67,73 After approximately age 20 to 30 years, the normal mean value for GFR declines with age in both men and women, with a mean decrease of approximately 1 mL/min/1.73 m2 per year. Thus, by age 70, the normal mean value is approximately 70 mL/min/1.73 m2. At all ages, the range of normal GFR is wide.
Data from NHANES III are shown in Figs 9 and 10; these include men and women in the general population, including those with chronic kidney disease.
Figure 10 |
|
Fig 10. Percentiles of estimated GFR regressed on age (NHANES III). GFR estimated from serum creatinine using MDRD Study equation based on age, gender, and race (see Part 10, Appendix 3). Age |
|
In part, the inclusion of women and individuals with chronic kidney disease may account for the slightly lower mean values observed in the NHANES III compared to the data from normal men in Fig 9.
Factors other than age also affect GFR. As shown in Table 24, GFR is slightly lower in young women than in young men. This difference appears to persist at older ages. Pregnancy has a major effect on GFR, with GFR reaching values of 140% of normal during the end of the second trimester.
Additional factors that may affect GFR to a lesser degree include: transient increases in GFR after a high protein meal, a lower GFR in individuals’ following a habitually low protein diet, and antihypertensive agents (effect on GFR varies by class of agent), especially in patients with chronic kidney disease.
Definition of decreased GFR (R, O). The Work Group defined decreased GFR as <90 mL/min/1.73 m2. The interpretation of decreased GFR varies depending on age, duration, and the presence or absence of markers of kidney damage.
The lower limit of normal GFR varies with age. For example, as shown in Table 25, GFR <90 mL/min/1.73 m2 would be abnormal in a young adult. On the other hand, a GFR of 60–89 mL/min/1.73 m2 could be normal from approximately 8 weeks to 1 year of age and in older individuals. It is possible that GFR 30 to 59 mL/min/1.73 m2 could also be normal in individuals at the extremes of age, in vegetarians, after unilateral nephrectomy or in an older individual. It is likely that a GFR <30 mL/min/1.73 m2 is abnormal at all ages other than neonates. For these reasons, the Work Group based the definition of chronic kidney disease solely on the level of GFR only in individuals with GFR <60 mL/min/1.73 m2, whereas individuals with GFR 60 to 89 mL/min/1.73 m2 were considered to have chronic kidney disease only if they also had a marker of kidney damage (see Table 12, p. S47).
Decreased GFR may be acute or chronic. An acute decrease in GFR does not necessarily indicate the presence of kidney damage. For example, it is well known that a brief period of mildly decreased blood flow to the kidneys or transient partial obstruction of the urinary tract may cause decreased GFR without kidney damage. However, a sustained decrease in blood flow or prolonged obstruction is often associated with kidney damage. Chronically decreased GFR is more often associated with kidney damage. The Work Group arbitrarily chose a cut-off value of greater than 3 months for the definition of chronic kidney disease.
As discussed earlier, individuals with decreased GFR should be evaluated for markers of kidney damage to determine whether they have chronic kidney disease and to determine the cause of reduced kidney function. Even if there is no evidence of kidney damage, individuals with chronically decreased GFR may be at increased risk for adverse outcomes (for example, toxicity from drugs excreted by the kidney, and acute kidney failure in a wide variety of circumstances).
Association of level of GFR with complications (S, R, C, O). Decreased GFR is associated with a wide range of complications in other organ systems, manifested by high blood pressure, laboratory abnormalities, and symptoms. Severity of complications worsens as level of GFR declines (Part 6, Guidelines 7 through 12). The Work Group defined categories of decreased GFR as mild (Stage 2, 60 to 89 mL/min/1.73 m2), moderate (Stage 3, 30 to 59 mL/min/1.73 m2), and severe (Stage 4, 15 to 29 mL/min/1.73 m2). Although these definitions are arbitrary, evidence compiled in later guidelines supports these broad categories and cut-off levels.
Prevalence of decreased GFR by age (S). The prevalence of decreased GFR is higher in the elderly (Table 26).
Approximately 14.9 million individuals ≥ 70 years (74.5%) of age have decreased GFR. As already stated, not all individuals with decreased GFR have kidney disease. The prevalence of persistent albuminuria by GFR level and age group have not been determined, preventing an accurate estimate of the prevalence of chronic kidney disease among the elderly.
The prevalence of decreased GFR is lower in children. The Schwartz formula was used to estimate GFR in children aged 12 to 19 years in the NHANES III database. The lowest 1% of children had GFR below approximately 100 mL/min/1.73 m2. Reliable estimates of prevalence of categories of decreased GFR (mild, moderate, or severe) in children are not available from NHANES III.
Kidney Failure
Definition of kidney failure (R, O). Kidney failure is defined as either (1) a level of GFR to <15 mL/min/1.73 m2, which is accompanied in most cases by signs and symptoms of uremia, or (2) a need for initiation of kidney replacement therapy (dialysis or transplantation) for treatment for complications of decreased GFR, which would otherwise increase the risk of mortality and morbidity. Some patients may need dialysis or transplantation at GFR ≥ 15 mL/min/1.73 m2 because of symptoms of uremia. The Work Group acknowledges that the level of GFR selected for this definition is arbitrary and may need to be modified based on advances in kidney replacement therapy.
End-stage renal disease (R). End-stage renal disease (ESRD) is an administrative term in the United States, based on the conditions for payment for health care by the Medicare ESRD Program, specifically the level of GFR and the occurrence of signs and symptoms of kidney failure necessitating initiation of treatment by replacement therapy. ESRD includes patients treated by dialysis or transplantation, irrespective of the level of GFR.
The KDOQI definition of kidney failure differs in two important ways from the definition of ESRD. First, not all individuals with GFR <15 mL/min/1.73 m2 or with signs and symptoms of kidney failure are treated by dialysis and transplantation. Nonetheless, such individuals should be considered as having kidney failure. Second, among treated patients, kidney transplant recipients have a higher mean level of GFR (usually 30 to 60 mL/min/1.73 m2) and better average health outcomes than dialysis patients. Kidney transplant recipients should not be included in the definition of kidney failure, unless they have GFR <15 mL/min/1.73 m2 or have resumed dialysis.
The Work Group anticipated that most kidney transplant recipients would be considered to have chronic kidney disease according to the proposed classification. First, GFR is lower in patients with a solitary kidney and is even lower in kidney transplant recipients because of toxicity from immunosuppressive agents used to prevent and treat rejection, such as cyclosporine and tacrolimus. Second, biopsy studies demonstrate pathologic damage due to acute and chronic rejection in virtually all transplant recipients, even if serum creatinine is normal. However, because markers of kidney damage are not sensitive to tubulointerstitial or vascular damage, it is likely that some kidney transplant patients will have GFR ≥60 mL/min/1.73 m2 without markers of kidney damage. Such patients would not be classified as having chronic kidney disease by the proposed classification. The Work Group would consider them to be at increased risk of chronic kidney disease. Thus, all patients with a kidney transplant would be considered either to have chronic kidney disease or to be at increased risk of chronic kidney disease.
Relationship of GFR to other measures of kidney function in kidney failure (S). A number of measurements, including GFR, have been used to quantify the level of kidney function among patients with kidney failure. The KDOQI Nutrition in Chronic Renal Failure Guidelines75 and Peritoneal Dialysis Adequacy Guidelines Update 200016 recommend the decision to initiate dialysis in adults be based on a combination of measurements of kidney function, as well as nutritional status. These guidelines are reproduced here:
Peritoneal Dialysis Adequacy Guideline 1: When to Initiate Dialysis—Kt/Vurea Criterion (Opinion) “Unless certain conditions are met, patients should be advised to initiate some form of dialysis when the weekly renal Kt/Vurea (Krt/Vurea) falls below 2.0. The conditions that may indicate dialysis is not yet necessary even though the weekly Krt/Vurea is less than 2.0 are:
A weekly Krt/Vurea of 2.0 approximates a kidney urea clearance of 7 mL/min and a kidney creatinine clearance that varies between 9 to 14 mL/min/1.73 m2. Urea clearance should be normalized to total body water (V) and creatinine clearance should be expressed per 1.73 m2 of body surface area. The GFR, which is estimated by the arithmetic mean of the urea and creatinine clearance, will be approximately 10.5 mL/min/1.73 m2 when the Krt/Vurea is about 2.0.”
Peritoneal Dialysis Adequacy Guideline 2 and Nutrition in Chronic Renal Failure Guideline 27: Indications for Renal Replacement Therapy (Opinion) “In patients with chronic kidney failure (e.g., GFR <15–20 mL/min) who are not undergoing maintenance dialysis, if protein-energy malnutrition (PEM) develops or persists despite vigorous attempts to optimize protein and energy intake and there is no apparent cause for malnutrition other than low nutrient intake, initiation of maintenance dialysis or a renal transplant is recommended.”
The CKD Work Group searched for studies of measures of kidney function, dietary intake, and nutritional status at the onset of kidney replacement therapy. The largest and most comprehensive study is the one reported in abstract by the MDRD Study Group.76 This study included 88 patients who were referred to their physicians by the MDRD Study investigators for initiation of dialysis because of symptoms or findings of uremia prior to the end of the study. Prescribed protein intake during the study was 0.6 g/kg/d, either as food or from food and a mixture of essential amino acids and ketoacids. The median interval from final GFR to initiation of dialysis in the study group was 89 days. Because these patients were participating in a clinical trial, the mean level of kidney function and nutritional status may be higher than in patients beginning dialysis in the general population. Tables 27 and 28 show measures of kidney function and nutritional status in these patients with kidney failure just prior to initiation of dialysis.
The comparison of measured GFR to other kidney function measurements is shown in Table 29.
These data show that estimated GFR provides only a rough approximation of other measures of kidney function. This provides additional justification for performing other measures of kidney function to assess the need for kidney replacement therapy, as recommended in the KDOQI Peritoneal Dialysis Guidelines.16 It was the opinion of the Work Group that these measurements should be obtained in patients with estimated GFR <15 mL/min/1.73 m2, since, as described below, few patients begin dialysis at higher levels of GFR.
Level of GFR at initiation of replacement therapy (S, C). Clinicians initiate replacement therapy based on the level of kidney function, presence of signs and symptoms of uremia, the availability of therapy, and patient or surrogate preferences. There is variability among individuals in the relationship of level of kidney function to signs and symptoms of uremia. Notably, there is variability within and among health care systems in the availability of therapy.
The level of GFR at the beginning of dialysis has been estimated in more than 90,000 patients in the United States between 1995 and 1997, using data collected on the Medical Evidence Report (HCFA Form 2728) and the MDRD Study prediction equation (Fig 11).77
Figure 11 |
|
Fig 11. Level of GFR at initiation of replacement therapy (USRDS). Data from Obrador et al.77 |
|
The mean (SD) level of serum creatinine was 8.5 (3.8) mg/dL. The mean (SD) level of GFR at initiation of treatment was 7.1 (3.1) mL/min/1.73 m2. The proportion of patients initiating dialysis with a predicted GFR of 10 to 15, 5 to 10, and <5 mL/min/1.73 m2 was 11%, 63%, and 24%, respectively; 98% of patients began dialysis with predicted GFR ![]() 15 mL/min/1.73 m2.
15 mL/min/1.73 m2.
Tables 30, 31, and 32 summarize other studies of the level of kidney function at initiation of dialysis.
Overall, the results of these studies are consistent with the data from the MDRD Study (Table 27) and the large study shown in Fig 11.
Factors associated with level of kidney function at initiation of dialysis (R). Timing of initiation of replacement therapy varies by modality, clinical characteristics, and sociodemographic characteristics. Patients who receive a pre-emptive transplant or who are started on peritoneal dialysis begin replacement therapy at higher mean levels of GFR than patients starting hemodialysis. Dialysis is initiated at higher mean levels of GFR among patients who are older, or who have diabetes, cardiovascular disease, and other comorbid conditions.
Prevalence of kidney failure (S). The incidence and the prevalence of reported ESRD have doubled in the past 10 years in the United States (Fig 2). Data from the 2000 Annual Data Report of the USRDS documents the incidence of ESRD in 1998 of more than 85,000, or 308 per million individuals per year at risk. The point prevalence of ESRD on December 31, 1998 was more than 320,000, or 1,160 per million population, of whom 72% were treated by dialysis (230,000 patients, or 835 per million population) and 28% had functioning kidney transplants (90,000 patients, or 325 per 100,000). The number of individuals with GFR <15 mL/min/1.73 m2 not on dialysis has not been estimated reliably.
The prevalence of kidney failure treated by dialysis varies by age. On December 31, 1998, there were approximately 75,000 adults over 70 years of age (97 per million) with kidney failure treated by dialysis, compared to approximately 1,800 children (2.1 per million).
There are a number of limitations to the proposed definition and classification of chronic kidney disease. The Work Group believes that these limitations should be identified, but does not think that they invalidate the proposal. Instead, these limitations should serve to stimulate further research to refine the definition and classification.
First, as described later in Guideline 6, the known markers of kidney damage are not sensitive, especially for tubulointersitial and vascular disease and for diseases in the kidney transplant. Thus, the prevalence of chronic kidney disease may be substantially higher than the Work Group has estimated, and recognition of patients with chronic kidney disease may be limited due to misclassification. Second, as described in Guideline 4, the MDRD Study prediction equation has not been validated extensively at levels of GFR ≥ 90 mL/min/1.73 m2; thus, it is difficult to estimate the level of GFR above 90 mL/min/1.73 m2, and it may be difficult to distinguish between Stage 1 and Stage 2 of chronic kidney disease. Third, as described earlier, the cause of age-related decline in GFR and high blood pressure is not known. Possibly, it may be due to chronic kidney disease. If so, it would be more appropriate to classify individuals with GFR 60 to 89 mL/min/1.73 m2 without apparent markers of kidney damage as having chronic kidney disease rather than “decreased GFR.” Fourth, the GFR cut-off values for Stages 3 to 5 have been selected based on limited data with respect to the relationship between complications and level of GFR. Further studies may permit refinement of these cut-off values. Fifth, the association of level of GFR with complications of chronic kidney disease does not prove a causal relationship between the two. Nonetheless, in many cases there is adequate evidence of a causal relationship, and even if there is not, the associations accurately describe the burden of illness associated with the severity of chronic kidney disease. Sixth, prevalence estimates for stages of chronic kidney disease and the associations of level of GFR with complications are based largely on an analysis of data from NHANES III that has not yet been peer-reviewed. However, the Work Group believes that Appendix 2 provides sufficient detail to evaluate the methods.
There are a large number of clinical applications of the proposed definition and stages of chronic kidney disease. An overall approach to evaluation and treatment of patients with chronic kidney disease is given in Guideline 2, and recommendations for individuals at increased risk of chronic kidney disease are given in Guideline 3. Clinical applications are also given at the conclusion of each subsequent guideline. Finally, additional recommendations for evaluation, diagnosis, and treatment of chronic kidney disease are given in Part 9.
Implementation of a new approach to the patient, classification of severity, and assessment of risk for chronic kidney disease will require appropriate professional, patient, and public education effort, as well as administrative and regulatory changes.
Professional, Patient, and Public Education
Components of the implementation plan, which determined the success of KDOQI, are under development and will be applied to these guidelines. They include: widespread dissemination and easy access to the guidelines; educational interactive programs aimed at health professionals, patients, providers, administrators, manufacturers, and policy makers; information tools and systems to facilitate adherence; development of clinical performance measures; incorporation of guidelines into continuous quality improvement programs; development of quality assessment instruments; and update and review of the pertinent literature on an ongoing basis.
Administrative and Regulatory Changes
Revision of Medicare forms and HCFA billing codes will be necessary. For example, classification of kidney disease by the International Classification of Disease (9th Edition) (ICD-9) is based on duration (acute versus chronic), diagnosis, clinical presentation, markers of damage, and kidney function impairment. The KDOQI classification proposes that both diagnosis and stage (severity) should be included in the classification of chronic kidney disease. This would facilitate using administrative databases for epidemiological and outcomes surveys.
The Workgroup acknowledges that the proposed definition and classification chronic kidney disease and stages is arbitrary and can be refined by further research.
The normal range for GFR was defined using a relatively small number of individuals. It would be useful to conduct a large cross-sectional study of GFR in general population, across the full range of age, gender, race, ethnicity, protein intake, with adjustment for other factors, including high blood pressure, diabetes, and other conditions that affect GFR. This study would permit validation of prediction equations based on serum creatinine or other filtration markers within the normal range of GFR.
The outcomes of individuals with various stages of chronic kidney disease are not defined. A cohort study of patients with chronic kidney disease would enable definition of the relationship between factors and outcomes of stages of chronic kidney disease. This would be particularly useful in defining the relationships among stages of chronic kidney disease, progression of chronic kidney disease, initiation and progression of cardiovascular disease, health service utilization, and barriers to care.
Age-related rise in blood pressure and decline in GFR may be responsible for a large number of individuals in Stage 3 (GFR 30 to 59 mL/min/1.73 m2). There are even more individuals with high blood pressure and decreased GFR (GFR 60 to 89 mL/min/1.73 m2), who have not been classified as having chronic kidney disease. It would be useful to conduct cross-sectional and cohort studies of elderly individuals with normal and abnormal blood pressure and GFR to assess the effect of high blood pressure and decreased GFR in this population.