Chronic kidney disease (CKD) is a worldwide public health problem affecting more than 50 million people, and more than 1 million of them are receiving kidney replacement therapy.1,2 The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative™ (NKF-KDOQI™) Clinical Practice Guidelines (CPGs) on CKD estimate that CKD affects 11% of the US population,3 and those affected are at increased risk of cardiovascular disease (CVD) and kidney failure. Kidney failure represents about 1% of the prevalent cases of CKD in the United States,3 and the prevalence of kidney failure treated by dialysis or transplantation is projected to increase from 453,000 in 2003 to 651,000 in 2010.3,4
Management of CKD is costly. The Medicare CKD stage 5 population nearly doubled in the last 10 years, and the CKD population expanded, as well. Together, they account for 16.5% of Medicare expenditures, nearly double that of 10 years ago, and the total costs for kidney disease now approach 24% of Medicare expenditures.4 A growing body of evidence suggests that some of the adverse outcomes of CKD can be prevented or delayed by preventive measures, early detection, and treatment.
NKF-KDOQI™ CPGs presently offer strategies to manage hypertension,5 dyslipidemia,6 bone disease,7 anemia,8 nutrition,9 and CVD10 in patients with CKD. The present Guideline extends the scope of the NKF-KDOQI™ CPGs by offering strategies to diagnose and manage the treatment of patients with diabetes and CKD.
PROBLEM OF DIABETES AND CKD
Diabetes is the leading cause of CKD in developed countries and is rapidly becoming the leading cause in developing countries as a consequence of the global increase in type 2 diabetes and obesity.33 In the United States, microalbuminuria is found in 43%, and macroalbuminuria, in 8% of those with a history of diabetes.3 Moreover, diabetes accounts for 45% of prevalent kidney failure, up from 18% in 1980.4
Substantial underdiagnosis of both diabetes and CKD leads to lost opportunities for prevention, and inadequate or inappropriate care of patients with diabetes and CKD may contribute to disease progression. Nevertheless, diabetes care has improved because the benefits of meticulous management have become widely accepted and the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins has increased in patients with diabetes.4 Even so, fewer than 1 in 4 patients with diabetes receives at least 1 hemoglobin A1c (HbA1c) test, at least 1 lipid test, and at least 1 glucose testing strip each year, reflecting the need for better assessment of these high-risk patients.4
GOALS OF CPG AND CPR PROCESS
This CPG seeks to improve outcomes in patients with diabetes and CKD by providing strategies for the diagnosis (Guideline 1) and management (Guidelines 3 to 5 and CPRs 1 to 4) of CKD in the setting of diabetes and for the management of diabetes in the setting of CKD (Guideline 2). The general treatment of diabetes is beyond the scope of this guideline, and it is comprehensively addressed in the American Diabetes Association (ADA) guidelines.34
As part of an evolution in the development of CPGs, the Work Group divided its recommendations, which are based on a systematic review of the literature, into a series of Guidelines and Clinical practice recommendations (CPRs). The Guidelines were based on a consensus within the Work Group that the strength of the evidence was sufficient to make definitive statements about appropriate clinical practice. When the strength of the evidence was not sufficient to make such statements, the Work Group offered CPRs based on the best available evidence and on expert opinion. As new data become available, the strength of the evidence for many of the CPRs may become sufficient for the CPRs to become CPGs, illustrating the need for recurring reviews and updates of this document. Many of the research recommendations proposed by the Work Group were developed with the goal of strengthening the evidence for the CPRs to determine whether they should become Guidelines in the future.
The term “definitive” must be used with caution, particularly in the context of CPGs. Uncertainty is an immutable element of all scientific research, and the establishment of a Guideline should neither preclude nor render unethical further inquiry. Rather, the establishment of guidelines represents an evolving process that seeks to ensure that each patient receives the best possible care within the context of presently available medical knowledge.
Scope
The target population of this CPG is patients with CKD stages 1 to 5, including dialysis and transplant patients. However, the emphasis is on stages 1 to 4 because the evidence in stage 5 is either lacking or addressed in other NKF-KDOQI™ Guidelines. Consideration is given to the diagnosis, impact, and management of diabetes and CKD in children, adults, the elderly, pregnant women, and different racial and ethnic groups.
The intended readers are practitioners who manage patients with diabetes and CKD, including, but not limited to, primary care providers, nephrologists, cardiologists, endocrinologists/diabetologists, physician’s assistants, nurse practitioners, nurses, dietitians, pharmacists, social workers, and diabetes educators. By reviewing scientific evidence from throughout the world, coordinating our efforts with guideline development processes elsewhere, and including in the Work Group experts from Latin America and Europe, as well as from North America, we believe this document has relevance beyond practitioners in North America.
An Epidemic
Nearly 21 million people in the United States, or 7% of the population, have diabetes, and about a third of those with diabetes are unaware they have the disease. About 5% to 10% of diabetes in the United States is type 1, which develops as a consequence of the body's failure to produce insulin. In some racial and ethnic groups, the proportion of cases attributable to type 1 diabetes is even less.11 Most cases of diabetes in the United States and elsewhere are type 2, which develops because of the body's failure to produce sufficient insulin and properly use the insulin it produces. Worldwide, 171 million people have diabetes.
Diabetes prevalence is increasing most rapidly in the developed countries and in developing countries undergoing transition from traditional to modern lifestyles.12,13 In the general US population, estimates from national surveys14 show an 8-fold increase in the prevalence of diagnosed diabetes between 1958 and 2000. The San Antonio Heart Study15 suggests an increasing incidence rate of type 2 diabetes is responsible, in part, for the increasing prevalence among Mexican Americans and for a borderline significant trend in non-Hispanic whites. The investigators attribute the greater prevalence of diabetes in this population more to the increasing incidence than to the decrease in cardiovascular mortality reported among people with diabetes nationally.16 Other factors responsible for the increasing prevalence of diabetes include changes in diagnostic criteria, increased public awareness, decreasing overall mortality, growth in minority populations, a dramatic increase in the magnitude and frequency of obesity, and the widespread adoption of a sedentary lifestyle.14 Most of the increase in diabetes prevalence is attributable to type 2 diabetes, and although much of this increase is occurring in adults, children and adolescents are increasingly affected. However, a worldwide increase in the incidence of type 1 diabetes also has been noted, particularly among children younger than 5 years.17
Projections of the future burden of diabetes in the US population suggest that the prevalence of diabetes will increase 165% between 2000 and 2050, with the greatest increases in the population older than 75 years and among African Americans.18 The global burden of diabetes is expected to double between 2000 and 2030, with the greatest increases in prevalence occurring in the Middle East, sub-Saharan Africa, and India.19 Moreover, development of type 2 diabetes during the childbearing years also will increase, primarily in the developing countries (CPR 3, Fig 27).19 Projections regarding the future burden of diabetes are based on increasing life expectancy, population growth, and progressive urbanization.20 Of growing concern is the belief that these estimates may be too low because they do not account for the increasing frequency and magnitude of obesity and other major risk factors for diabetes.
As the population of patients with diabetes of long duration grows, reports of a dramatically increasing burden of diabetic kidney disease (DKD) are appearing from developed countries,21 as well as from Africa,22,23 India,24 the Pacific Islands,25 and Asia,26,27 where infectious disease previously posed the greatest threat28 (see CPR 3). Increased risk and more rapid progression of DKD29,30 also have been reported in immigrants from developing to developed countries.31,32
Obesity and Inactivity
Obesity is one of the strongest determinants of diabetes and is a consequence of interactions between genetic susceptibility, cellular metabolism, eating behavior, culture, level of physical activity, and socioeconomic status. Because obesity is a major determinant of diabetes and other chronic diseases, an assessment of obesity should be part of the routine clinical examination of every patient. General measures of obesity (BMI, weight, and percent body fat) and measures of central fat distribution (waist circumference, waist-hip ratio, waist-thigh ratio, and waist-height ratio) predict the development of type 2 diabetes in prospective studies, regardless of age or ethnicity.47-54 Although a strong relationship exists between the quantity of intra-abdominal fat and diabetes, BMI remains an excellent predictor of diabetes and is not improved significantly by combining it with other measures of general adiposity or body fat distribution.55 In the kidney, obesity is associated with glomerular hyperfiltration and an increase in transcapillary hydraulic pressure,56,57 hemodynamic changes that may accelerate the development and progression of DKD in obese people with diabetes. Hence, CPR 2 was developed to address the issue of obesity and encourage further investigation.
One of the primary determinants of obesity is physical inactivity, and a physically active lifestyle is associated with a lower incidence of type 2 diabetes in several prospective studies.58-62 Recent clinical trials provide compelling evidence that increased physical activity, combined with dietary modification and weight loss, prevents diabetes regardless of age or ethnicity.63-65 The Diabetes Prevention Program demonstrated that a lifestyle-modification program that included a 7% weight loss and at least 150 minutes of moderate physical activity per week was associated with a 58% reduction in the incidence of diabetes over nearly 3 years in people with impaired glucose tolerance compared with placebo.63 Because the lifestyle changes worked equally well in all racial/ethnic groups, they should be applicable to high-risk populations worldwide. This approach to diabetes prevention provides the most cost-effective means for reducing the projected increase in the incidence of diabetes and its complications, including DKD.66
Ethnicity
In the United States, the burden of diabetes is borne disproportionately by ethnic and racial minorities, including African Americans, Hispanics, and American Indians. The higher rates of diabetes in these populations relative to non-Hispanic whites are associated with a high rate of DKD, as described in CPR 3. The particularly high predisposition to diabetes is possibly on a genetic basis, when individuals are exposed to adverse conditions or rapid economic transition. Worldwide, populations of developing countries appear to be at increased risk of developing diabetes during the coming decades, perhaps for many of the same reasons.
Economic transition may be the predominant risk factor for diabetes in many developing countries. People who successfully undergo economic transition—those who migrate to cities and take industrial jobs that pay well—experience an increase in socioeconomic status and greater access to food. In India, for example, higher socioeconomic status increases the risk of diabetes.67 The same is true among Hispanics in the United States.68 Conversely, transition to higher socioeconomic status has the opposite effect in African Americans69; a finding that may be explained in part because higher socioeconomic status generally is associated with better education, greater acculturation, and the resources to make healthier food choices.70 Therefore, although populations in rapid economic transition often are at increased risk of diabetes, proper education may mitigate or prevent the increase in the risk of diabetes often associated with this transition.
Extremes of Age
The current epidemic of obesity in children and adolescents in many parts of the world has created an epidemic of type 2 diabetes in these age groups. Although type 1 diabetes is the predominant form of diabetes in children worldwide, it is likely that type 2 diabetes will soon become the most prevalent form in many ethnic groups.71,72 Many children with type 2 diabetes are obese at diagnosis, have a strong family history of type 2 diabetes, and are the offspring of mothers with gestational diabetes. Among American Indians aged 15 to 19 years nationwide, the prevalence of diagnosed diabetes increased by 69% from 1990 to 1998, but remained unchanged in those younger than 15 years.73 In Japan, a 10-fold increase in the incidence of type 2 diabetes was reported during 20 years of follow-up in children initially aged 6 to 12 years, and a 2-fold increase was reported among those initially aged 13 to 15 years, coinciding with a secular increase in the prevalence of obesity.74,75
The proportion of children exposed to diabetes in utero also may be increasing as more women develop diabetes during their childbearing years. In Pima Indians, a doubling of the percentage of childhood diabetes during the past 30 years is attributed to an increasing frequency of intrauterine exposure to diabetes.76 Observations in Pima children born since 1965 indicate that offspring of mothers with diabetes have a greater prevalence of obesity throughout childhood and a much greater prevalence of type 2 diabetes.77 Although only 3% of type 2 diabetes develops before 20 years of age,78 those who develop diabetes in childhood and adolescence are affected disproportionately in early adulthood by the microvascular and macrovascular complications of diabetes, including DKD,79,80 as described in CPR 3.
The World Health Organization (WHO) Multinational Study of Vascular Disease in Diabetes81 reported that American Indians from Arizona and Oklahoma who had type 2 diabetes diagnosed before 30 years of age had a higher age-adjusted incidence rate of kidney failure during a mean follow-up of 9.5 years than the overall Native American population with diabetes.82 A study of long-term microvascular and macrovascular complications in Japanese subjects with onset of type 2 diabetes before 30 years of age83,84 found that 5% of the subjects had CKD stage 5 after 20 years' duration of diabetes, and 23% of those who also had proliferative retinopathy progressed to dialysis by a mean age of 35 years. Premature atherosclerotic vascular disease, including cerebrovascular disease and CVD, was the leading cause of death in this population and was related largely to poor glycemic control and progression to CKD stage 5. These complications have a significant economic and public health impact because they will affect those with youth-onset diabetes during their peak productive years.
Diabetes is a major cause of morbidity and mortality in the aging population. At least 20% of people older than 65 years have diabetes,34 and the greatest increase in diabetes prevalence in the coming decades will occur in those older than 75 years.18 The elderly are particularly prone to the cardiovascular complications of diabetes. CVD develops in the 2 years before initiation of kidney replacement therapy in more than 90% of patients aged 75 years and older with kidney failure and diabetes. Congestive heart failure is the most common cardiac condition among elderly patients with diabetes and CKD stage 5, affecting 71% of patients, followed closely by ischemic heart disease at 67%.21
Other comorbidities also are more prevalent in the elderly, and intensive management of these patients may pose greater risks because hypotension and hypoglycemia occur more frequently than in younger people. Although medicines for hyperglycemia, hypertension, and dyslipidemia can be used in the elderly, as in other patients with diabetes and CKD, they should be started at lower doses and carefully titrated while monitoring for responses and side effects (see CPR 3). The ADA, in collaboration with the American Geriatric Society, has published evidence-based guidelines for the management of geriatric patients with diabetes.85
Pregnancy
The effect of pregnancy on diabetes and CKD is examined in CPR 3. Diabetes during pregnancy is associated with an increased risk of adverse maternal and neonatal outcomes. The frequency of diabetes during pregnancy is increasing in developed countries primarily because of increasing obesity among women of childbearing age. Early diagnosis of diabetes during pregnancy may be an important factor in improving outcomes in these mothers and their offspring. Nevertheless, much of the projected increase in diabetes prevalence during the childbearing years will occur in developing countries,19 where resources for identifying and managing the diabetic pregnancy are limited.
Whereas the maternal complications of diabetes are well known, there is increasing evidence that the effects on the fetus are more extensive than previously thought. In addition to increased rates of macrosomia, congenital malformations, and perinatal mortality, the offspring of mothers with diabetes are prone to obesity and diabetes at a young age, leading to a vicious cycle of increasing frequencies of diabetes in successive generations.86 In the Pima Indians, for example, the proportion of children exposed to diabetes in utero increased nearly 4-fold during the past 30 years.76 The increased frequency of exposure to maternal diabetes was associated with a doubling of the number of cases of diabetes attributable to that exposure.76 Moreover, the odds of having increased urinary albumin excretion was nearly 4 times as high in the offspring with diabetes who were exposed to diabetes in utero than in those exposed to a normal intrauterine environment.87 These findings suggest that a diabetic pregnancy contributes not only to the increase in diabetes prevalence worldwide, but also to the increase in DKD among those who develop diabetes as a consequence of this exposure. Whether strict glycemic control during a diabetic pregnancy will reduce the frequency of diabetes and kidney disease in the offspring is unknown. Management of young obese women who desire to become pregnant should focus on preventing or at least delaying the onset of diabetes until after the childbearing years.
Vascular Target-Organ Complications Cause Much Morbidity and Mortality
Diabetes is associated with numerous vascular and nonvascular complications, and the vascular complications—which include CVD, peripheral vascular disease, stroke, retinopathy, neuropathy, and DKD—are responsible for most of the morbidity and mortality attributable to diabetes. The frequency of disability in people with diabetes offers an indirect means of assessing the morbidity associated with various vascular complications. Ischemic heart disease, stroke, and peripheral vascular disease increase the risk of mobility-related disability in older adults with diabetes in the United States by 2- to 3-fold relative to those without diabetes.88,89 The Third National Health and Nutrition Examination Survey (NHANES III) found that in the United States, 25% of adults older than 60 years with diabetes cannot walk one quarter of a mile, climb 10 stairs, or do housework, and half of those in this age group have some difficulty performing these tasks.88 Peripheral neuropathy often leads to greater limitations in performing the personal care activities of daily living, but has less impact on mobility.89 Diabetes is the leading cause of visual deficits in developed countries among people younger than 60 years,90,91 and visual impairment or blindness can lead to disability affecting both mobility and daily living activities.
One measure of population health and morbidity, the disability-adjusted life-year (DALY), provides an estimate of the length of life lost to premature death and the time spent in an unhealthy state. This measure is computed for the US population from data collected by the NHANES, the National Health Interview Survey, and several other nationally representative health surveys.92 Diabetes is the 9th leading cause of DALYs among women and the 12th leading cause among men in the United States. African Americans, Hispanics, Asians, Pacific Islanders, and American Indians have the highest DALYs related to diabetes, in keeping with their greater prevalence and earlier onset of diabetes. The impact of diabetes on DALYs and other health outcomes in these minority populations also may be affected by disparities in their health that result from their social, political, and economic disadvantage.92
In the United States, the death rate in people with diabetes is twice that of people without diabetes, and the major cause of the increased death rate among those with diabetes is CVD (vide infra).93 Moreover, nearly all the excess mortality in both type 194 and type 295 diabetes is found in people with proteinuria. The WHO Multinational Study of Vascular Disease in Diabetes96 reported that proteinuria was associated with significantly increased mortality from kidney failure, CVD, and all other causes of death. Kidney and cardiovascular mortality ratios associated with proteinuria were similar for both types of diabetes, although people with type 1 diabetes were more likely to die of kidney failure than those with type 2 diabetes.96
Terminology for the Kidney Disease of Diabetes
New terminology to describe kidney disease attributable to diabetes is introduced in the Diabetes and CKD guidelines. The purpose of this terminology is to clarify communication among patients, caregivers, and policy makers. For this purpose and for consistency with CKD classification, the term DKD is proposed for a presumptive diagnosis of kidney disease caused by diabetes. Although kidney biopsy is required to diagnose diabetic glomerulopathy definitively, careful screening of diabetic patients can, in most cases, identify persons most likely to have diabetic glomerulopathy without the need for kidney biopsy (see Guideline 1). The term “diabetic nephropathy” should be replaced by DKD. The term diabetic glomerulopathy should be reserved for biopsy-proven kidney disease caused by diabetes.
The goals of Guideline 1 are to facilitate identification of patients with kidney disease presumed to be caused by diabetes and distinguish them from those who should have further investigation for a different diagnosis, which may alter treatment plans. Most clinical studies of kidney disease in diabetes include patients with low glomerular filtration rate (GFR) and/or proteinuria, with a presumptive diagnosis of DKD. However, in practice, few patients have biopsy-proven DKD. Nevertheless, it would be useful to distinguish patients with CKD that is presumed to be caused by diabetes (DKD) from those with CKD from other causes on a clinical basis. DKD is based historically on the finding of proteinuria in a person with diabetes. With the development of more sensitive assays specific for albumin, DKD is now defined, in part, by increased urinary albumin excretion, which is divided arbitrarily into: (1) microalbuminuria, a modest elevation of albumin thought to be associated with stable kidney function, but a greater risk of macroalbuminuria and kidney failure; and (2) macroalbuminuria, a higher elevation of albumin associated with progressive decline in GFR, an increase in systemic blood pressure, and a high risk of kidney failure (Guideline 1, Table 6). However, these generalizations do not apply in all cases because people with normal urinary albumin excretion may have advanced DKD, whereas those with microalbuminuria may have either substantial or no pathological evidence of kidney damage. Moreover, because of the high prevalence of diabetes in the population, some individuals with diabetes may have other types of CKD. Nevertheless, in most cases, clinical measures may be used to diagnose DKD.
Screening and Diagnosis
Most professional societies concerned with diabetes and kidney disease now advocate screening for microalbuminuria in patients with diabetes, and the suggested screening plan, adapted from the ADA guideline, is shown in Guideline 1, Fig 6.34,35 The Work Group supports these screening recommendations while recognizing the need for further studies to define the impact of microalbuminuria detection on hard clinical end points (see Guideline 1). Screening should begin after 5 years of type 1 diabetes and at the diagnosis of type 2 diabetes because of the inability to establish the onset of type 2 diabetes with certainty. Because urinary albumin excretion has an intraindividual coefficient of variation (CV) of approximately 40%,36 multiple positive test results are required for classification. Definitions of DKD by albuminuria and stage are shown in Guideline 1, Table 6.
Evidence for the usefulness of estimated GFR (eGFR) alone as a screening test for CKD in patients with diabetes is less secure. Many patients with diabetes and CKD may have elevated or high-normal GFRs, particularly in the early years after diagnosis. Therefore, markers of kidney damage are required to detect early stages of CKD; eGFR alone can only detect CKD stage 3 or worse (Guideline 1, Table 6).
Diabetes May Coexist With Other Causes of CKD
Because diabetes is a common condition, coincidence with other nondiabetic CKD is relatively frequent. Accordingly, evaluation of a person with atypical features should, in selected cases, include additional diagnostic testing, depending on the clinical presentation. Care should be used in determining the appropriate diagnostic tests because administration of radiographic contrast, with or without angiography, may pose greater risks in people with diabetes and CKD than in others (see Guideline 1).
Refractory hypertension and/or a significant reduction in kidney function after renin-angiotensin system (RAS) blockade should prompt consideration of renal artery stenosis because generalized vascular disease is common in diabetes. Patients with diabetes and CKD in whom refractory hypertension is suspected should be evaluated, preferably without radiocontrast, to assess whether arterial stenosis is present. Current noninvasive modalities to screen for arterial stenosis that do not include use of radiocontrast agents include magnetic resonance angiography and duplex Doppler ultrasonography. Captopril nuclear renal scans are not recommended because sensitivity of these scans is low in patients with decreased GFR or bilateral renal artery stenosis. In selected cases, imaging of the renal arteries may be undertaken with carbon dioxide or gadolinium angiography to avoid radiocontrast agents.
Hypertension associated with unilateral renal artery stenosis may be treated with medicine (preferably an ACE inhibitor or ARB) with the option of revascularization, usually by percutaneous angioplasty and stent placement. Treatment of bilateral renal artery stenosis, or unilateral renal artery stenosis in an individual with a single functioning kidney, may require revascularization to both control hypertension and prevent loss of kidney function. However, whether revascularization of unilateral or bilateral renal artery stenosis adds benefit to optimal medical management is uncertain. Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL), a randomized trial sponsored by the National Institutes of Health (NIH), is addressing this key issue and should provide important direction for the management of renal artery stenosis in the future.
A number of systemic diseases that require specific therapy may occur in patients with diabetes. These diseases may present with a slow progressive decline in kidney function or a rapid decrease and may affect the kidney in various ways. Systemic diseases mostly likely to be confused with DKD are those that cause mild to moderate proteinuria and a slow progressive decrease in eGFR. Differentiation of these diseases requires clinical suspicion and appropriate diagnostic testing. It is the opinion of the Work Group that in the absence of another identifiable and treatable cause of kidney disease, patients with diabetes and CKD should be treated as if they have DKD (see Guideline 1).
DIABETES, CKD, AND CVD
Diabetes and CKD: A High-Stakes Combination for Cardiovascular Complications and Death
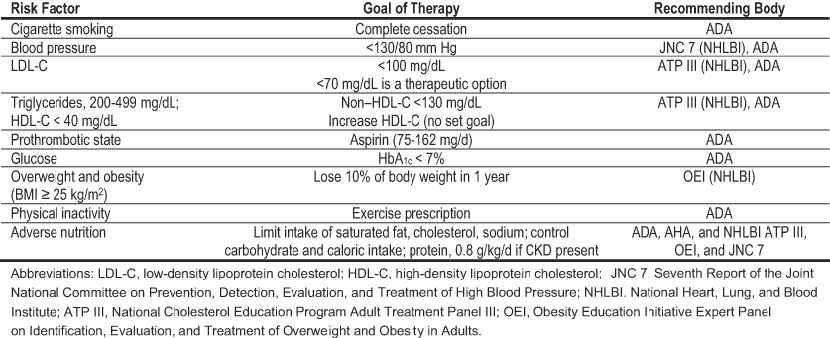
Diabetes is one of the most important risk factors for CVD. The risk imparted by diabetes has been viewed as a CVD equivalent because the likelihood of future events may approach that of people without diabetes who have already had a myocardial infarction.37 Such observations have led to recommendations from both the ADA and the American Heart Association (AHA) for intensive cardiovascular risk factor management in people with diabetes (Table 1).34,38 CKD also imparts an extremely high risk of CVD. The NKF and the AHA have recently issued guidelines and scientific statements recommending that people with CKD be considered in the highest risk category for CVD.3,39 For those with both diabetes and CKD, the outlook is far worse than for either condition alone because the combination is one the most powerful predictors of major adverse cardiovascular events and death. The relationship between CKD severity and risk is continuous. People with diabetes and microalbuminuria have twice the CVD risk of those with normoalbuminuria,40 and as albuminuria increases and GFR decreases, CVD risk increases progressively.41-43 In an analysis of patients with type 2 diabetes from the UK Prospective Diabetes Study (UKPDS), rates of death and progression to macroalbuminuria were equal at the microalbuminuric stage.41 However, at the macroalbuminuric stage, the death rate outpaced the rate of kidney disease progression (Fig 2). More people who reach CKD stage 3 will die, primarily of CVD, than progress to kidney failure, especially if they also have diabetes.3,44
Table 1. Goals for CVD Risk Factor Management in Patients With Diabetes34,38

The scope of this review of relationships among diabetes, CKD, and CVD is relevant primarily to people with CKD stages 1 to 4. Specific recommendations for CKD stage 5 are provided in the NKF-KDOQI™ Guidelines for CVD in Dialysis Patients.10
Intensive Risk Factor Management for Prevention of CVD
Risk factor management is the cornerstone of therapy for CVD in patients with diabetes. In the present NKF-KDOQI™ Guidelines on Diabetes and CKD, intensive management of hypertension, hyperglycemia, and dyslipidemia is emphasized. Although evidence was reviewed primarily for effects on kidney outcomes, the conclusions regarding therapeutic goals and choices of agents are strikingly similar to recommendations from the ADA and AHA for prevention and treatment of CVD (Table 1). These similarities likely reflect underlying pathological mechanisms common to both diabetic microvascular and macrovascular complications.

Figure 2. Annual Transition Rates with 95% Confidence Intervals Through the Stages of Nephropathy and to Death from any Cause.
Reprinted with permission.41
Table 2. Diagnostic Testing for Coronary Heart Disease in Diabetes34,38
Recommendations for treatment of dyslipidemia in patients with diabetes and CKD are based on CVD risk reduction. The current state of evidence is insufficient to recommend treatment of dyslipidemia for preservation of kidney function. The recommendation to treat with statins in diabetes and CKD stages 1 to 4 was based primarily on large prospective studies of patients with diabetes without markedly decreased kidney function and on a post hoc analysis from the Pravastatin Pooling Project (PPP).97-99 In the PPP, people with diabetes and CKD had the greatest risk of CVD death, myocardial infarction, or revascularization procedures compared with those with either condition alone or neither condition.99 They also had the largest absolute risk reduction with statin therapy (Guideline 4, Fig 19). Despite these impressive results, the evidence was considered moderate by the Work Group because it was based largely on this post hoc analysis. Prospective randomized trials are needed to confirm or refute these results and increase confidence in the data. This issue is especially germane considering results of the Deutsche Diabetes Dialyse Studie (4D), which showed no overall benefit on the primary outcome of major CVD events after initiating atorvastatin treatment in patients with type 2 diabetes receiving hemodialysis therapy (Fig 5).100 Based on results of the 4D, initiation of statin therapy is not recommended for people with type 2 diabetes on hemodialysis therapy who do not have a specific cardiovascular indication for treatment. Ongoing studies evaluating lipid-lowering therapies for CVD risk reduction in people with diabetes and CKD are critically important to define optimal treatment strategies. Considering the very different conclusions of the PPP and 4D, the window of opportunity for statin therapy to reduce CVD risk in patients with diabetes and CKD remains to be defined.
Evaluation for Coronary Heart Disease
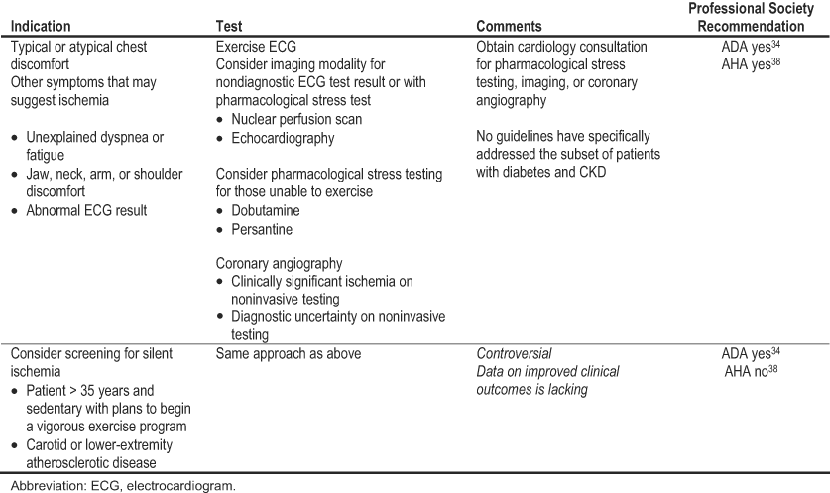
Cardiac ischemia is a predominant form of CVD leading to major complications and death in people with diabetes and CKD. A body of research on evaluation for coronary heart disease has lead to evidence-based CPGs from major professional societies. Coronary artery revascularization procedures are warranted in some patients. To identify appropriate candidates, further diagnostic testing should be performed based on specific clinical indications (Table 2). The recommendations from the ADA and AHA apply to people with diabetes in general.34,38 No guidelines have been developed for the subset of patients with diabetes and CKD. In the opinion of the Work Group, these recommendations reasonably can be extrapolated to most patients who have both diabetes and CKD stages 1 to 4, especially considering that their CVD risk is amplified over that of diabetes alone.
The specific clinical indications for noninvasive testing for coronary heart disease include typical or atypical chest discomfort or other symptoms of possible ischemia (eg, unexplained dyspnea or fatigue or jaw, neck, arm, or shoulder discomfort).34,38 An electrocardiogram (ECG) should be included in the CVD risk assessment of all people with diabetes and repeated for any symptoms suggestive of cardiac ischemia. If an ECG result is abnormal, further diagnostic testing should be considered. Whether asymptomatic people with diabetes should undergo diagnostic testing for coronary heart disease is controversial. At present, data that such an approach improves prognosis beyond risk factor assessment and management are lacking. However, patients with diabetes and silent ischemia, especially if accompanied by cardiac autonomic neuropathy, have a poor prognosis. Therefore, the ADA recommends that screening for silent ischemia may be considered for certain high-risk characteristics: 35 years or older and sedentary with plans to begin a vigorous exercise program, and carotid or lower-extremity atherosclerotic disease.34 The presence of traditional CVD risk factors did not predict silent ischemia in the cross-sectional Detection of Ischemia in Asymptomatic Diabetics (DIAD) study.101 Therefore, the ADA no longer recommends screening of asymptomatic people with diabetes on the basis of risk factor clustering (≥2 risk factors).34 When the longitudinal component of DIAD is completed, data will be available on the relationship between abnormal cardiac nuclear perfusion imaging results and clinical events. In the meantime, the AHA does not endorse diagnostic testing for coronary heart disease in asymptomatic patients with diabetes because of the lack of evidence to support the benefits of testing on clinical outcomes.38
A noninvasive approach to diagnostic testing is preferred as the first step in evaluating coronary heart disease.34,38 However, as discussed next, an initial invasive approach may be necessary in those with acute ischemic syndromes. According to the AHA and ADA, stress testing with exercise ECG should be the initial noninvasive strategy.34,38 Cardiology consultation should be obtained if evaluation beyond exercise ECG testing is necessary. Those who have nondiagnostic exercise ECG test results may benefit from the addition of an imaging modality (nuclear perfusion scan or echocardiography) to the exercise procedure.38 However, the NKF-KDOQI™ Guidelines for CVD in Dialysis Patients do not recommend exercise ECG testing because of poor exercise tolerance in general and a high prevalence of left ventricular hypertrophy in dialysis patients.10 Many patients with advanced CKD are likely to be similarly affected. Therefore, for these patients or others who cannot exercise adequately, pharmacological stress testing (dobutamine or persantine) with imaging is indicated.10,34,38 Coronary angiography may be performed if evidence for clinically significant ischemic heart disease is detected or for diagnostic uncertainty. As detailed in Guideline 1, people with diabetes and CKD are at high risk of acute kidney failure due to radiocontrast-induced nephropathy (RCN). Whenever possible, preventive strategies should be used to mitigate this risk (Guideline 1, Table 18). Nevertheless, considering the extremely high CVD risk in patients with diabetes and CKD, angiography should not be avoided if clinical indications for the invasive assessment and/or treatment of ischemic heart disease are present.
Medical Management of Coronary Heart Disease
RAS Inhibition. In people with diabetes and CKD, RAS inhibition is beneficial for the management of coronary heart disease and associated complications, as well as for treatment of hypertension. ACE inhibitors and ARBs reduce mortality after acute myocardial infarction,102,103 and when used alone or in combination, these agents are equally beneficial for improving survival and reducing CVD events after myocardial infarction complicated by left ventricular dysfunction.103 Patients with diabetes benefit at least as much as those without diabetes.103 Similarly, in people with diabetes with chronic coronary heart disease and without left ventricular dysfunction, ACE inhibition reduces CVD death, myocardial infarction, and stroke.104,105 Therefore, RAS inhibition is recommended for treatment of acute myocardial infarction and for chronic coronary heart disease in patients with diabetes.34,38,102 Recent post hoc analyses indicate that ACE inhibition is likely to be at least as efficacious at reducing CVD risk in people with and without diabetes and CKD, as it is for others with coronary heart disease.106,107 As detailed in Guideline 3, data regarding effects of ACE inhibition for treatment of hypertension on DKD progression in type 2 diabetes are not as strong as in type 1 diabetes. However, given their proven cardiovascular benefits and the shared properties of ACE inhibitors and ARBs in inhibiting the RAS, either type of agent should be strongly considered for people with diabetes and CKD because they reduce the risk of both CVD events and progression of kidney disease.
β-Blockers. β-Blockers are another therapeutic class with unique benefits for CVD. Among people with and without diabetes who have had a myocardial infarction, the American College of Cardiology (ACC)/AHA guidelines recommend use of β-blockers because they reduce the risk of death, reinfarction, and recurrent ischemia.102 β-Blockers also are recommended by the AHA for the long-term treatment of patients with diabetes and left ventricular dysfunction, but the basis of this recommendation is not as firm as for ACE inhibition.38 Although β-blockers may mask symptoms of hypoglycemia or exacerbate glucose intolerance, these side effects usually are manageable. In addition, β-blockers vary in their effects on glycemia. For example, the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) trial demonstrated that in the presence of an ACE inhibitor or ARB, carvedilol stabilized glycemic control and improved insulin resistance to a greater extent than metoprolol in patients with type 2 diabetes and hypertension.108 Therefore, considering their substantial cardiovascular benefits, the AHA recommends that β-blockers not be avoided in patients with diabetes for fear of side effects.38 Based on their remarkably high CVD risk, the Work Group recommends that the ACC/AHA and AHA guidelines regarding use of β-blockers also be applied to the subset of patients with diabetes and CKD.
Aspirin. Platelet inhibition with aspirin is strongly encouraged for the prevention and management of ischemic heart disease in patients with diabetes.34,38 In the opinion of the Work Group, people with diabetes who have CKD should receive aspirin as part of a multifaceted approach to treatment, as outlined in CPR 2.
Intensive Glycemic Control in Acute and Long-Term Care Settings. Glucose-insulin-potassium infusion (GIK) and intensive glycemic control are advocated for reducing mortality risk after acute myocardial infarction or with critical illness (especially after cardiac surgery) in people with and without diabetes.109,110 Although the ACC/AHA and the ADA recommend normalization (or nearly so) of blood glucose levels within 24 to 48 hours after myocardial infarction, more recent evidence does not substantiate this approach.34,102 Benefits of GIK therapy were described in relatively small studies or in meta-analyses in which the reduction in mortality risk had wide confidence intervals (CIs), indicating uncertainty in the conclusions.111 Recently, the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation (CREATE) and the Estudios Cardiologicas Latin America Study Group (ECLA) formally merged into a single trial, CREATE-ECLA, that randomly assigned more than 20,000 patients with acute myocardial infarction to receive GIK therapy or not.112 In this large trial, no benefits on death or reinfarction rates were observed after 30 days in the group as a whole or in predefined subgroups, including those with diabetes. Similarly, survival benefits of intensive insulin therapy in patients with critical illness were not substantiated in patients admitted to a medical intensive care unit irrespective of diabetes status, CVD, or kidney disease diagnosis.113 Although a subgroup analysis of patients who remained in the intensive care unit more than 3 days suggested a survival benefit, these patients could not be identified prospectively. Furthermore, in a larger follow-up study of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study, DIGAMI 2, the survival benefit of intensive glycemic control after myocardial infarction in patients with diabetes was not confirmed.114
Hypoglycemia is a well-recognized complication of GIK and intensive insulin therapy in the acute care setting. As discussed in Guideline 2, patients with CKD are at particularly high risk of hypoglycemia and associated morbidities with intensive regimens for glycemic control. Therefore, the position of the Work Group is that current evidence does not support routine use of intensive glycemic control in acute care settings, including myocardial infarction, for patients with diabetes and CKD.
Whether long-term intensive control of glycemia reduces CVD risk has long been debated. Recent data from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study indicate reduced rates of death, myocardial infarction, and stroke as many as 11 years after intensive management of type 1 diabetes has ceased (Fig 3).115 Reduction in these major adverse CVD events was mediated in part by reduction in incidence of DKD. In the UKPDS trial, intensive glycemic control in general did not decrease the risk of myocardial infarction. However, in a subset of overweight patients who received metformin, the rate of myocardial infarction was reduced.116 The Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) suggested that pioglitazone may reduce all-cause mortality, myocardial infarction, and stroke in patients with type 2 diabetes.117 In a post hoc analysis of people undergoing percutaneous coronary intervention (PCI) in the Prevention of Restenosis with Tranilast and Its Outcomes (PRESTO) trial, metformin use was associated with reduced risk of myocardial infarction and death in people with type 2 diabetes.118 Therefore, emerging data indicate that intensive glycemic control reduces the risk of CVD events and death, but the benefits appear to be primarily in long-term, rather than acute, intensive glycemia management. In type 2 diabetes, insulin-sensitizing agents may be beneficial for reducing CVD event rates. Prospective controlled trials should be conducted to confirm these observations. Importantly, caution is advised with use of metformin in patients with CKD, as discussed in Guideline 2. Although studies regarding intensive glycemic control and CVD in people with diabetes and CKD are nonexistent, the available data provide further support for the goal of reaching an HbA1c level less than 7% or as close to normal as possible without excessive episodes of hypoglycemia.
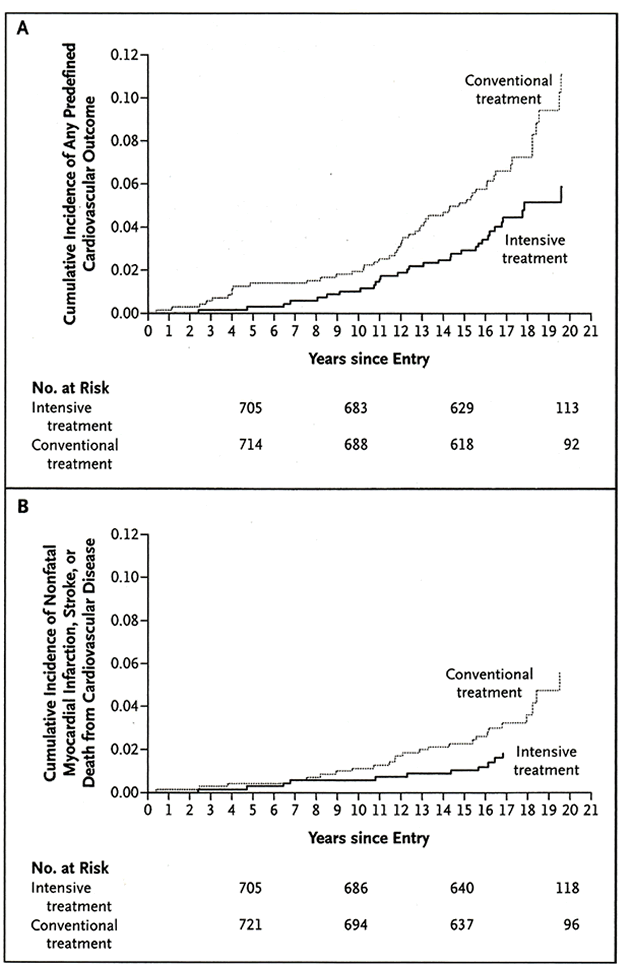
Figure 3. CVD Outcomes by Treatment Assignment in DCCT/EDIC.
CVD outcomes by treatment assignment in DCCT/ EDIC. (A) Cumulative incidence of the first of any of the predefined CVD outcomes. (B) First occurrence of nonfatal myocardial infarction, stroke, or death from CVD. Compared with conventional treatment, intensive treatment reduced the risk of (A) any predefined CVD outcome by 42% (95% CI, 9% to 63%; P = 0.02) and (B) first occurrence of nonfatal myocardial infarction, stroke, or death from CVD by 57% (95% CI, 12% to 79%; P = 0.02). Reprinted with permission.115
Reperfusion and Revascularization for Coronary Heart Disease
Acute Ischemic Syndromes. In virtually all aspects, management of acute myocardial infarction is similar for patients with and without diabetes.38 Reperfusion therapies for acute ST-segment elevation myocardial infarction are founded on a strong evidence base and have become the standard of care because deaths and subsequent major adverse cardiovascular events are reduced.102 Coronary artery reperfusion may be accomplished by using either PCI or fibrinolytic therapy. Where acute PCI is readily available with expert prompt intervention (within 90 minutes of first medical contact), this approach provides superior results compared with fibrinolysis.102 However, when acute PCI is not available, fibrinolysis should be used as the initial treatment strategy (within 12 hours of symptom onset) if contraindications do not exist (eg, history of intracranial hemorrhage, closed head or facial trauma, or ischemic stroke within the past 3 months; uncontrolled hypertension; bleeding diathesis; or aortic dissection).102 For acute coronary syndromes (unstable angina or non-ST-segment elevation myocardial infarction), medical management, including aspirin, heparin, glycoprotein IIb/IIIa inhibitors, β-blockers, and ACE inhibition, are indicated, usually along with coronary angiography and PCI.119
Evidence to guide treatment of patients with CKD is sparse. Despite their high risk of death and complications from myocardial infarction or acute coronary syndromes, those with CKD are less likely to receive reperfusion or other recommended therapies.120-123 Suboptimal approaches to managing acute cardiac ischemic syndromes in the CKD population may result from fear of such complications as acute kidney failure or bleeding, among others. However, when recommended therapies have been given to people with CKD, risk of death was decreased in observational studies.121-123 Data for the subset of patients with both diabetes and CKD do not exist. Clearly, this population should be included in future clinical trials of treatment for acute cardiac ischemic syndromes to define benefits and risks. In the meantime, the opinion of the Work Group is that the current standard of care for myocardial infarction and acute coronary syndromes, including PCI, fibrinolysis, antiplatelet strategies, and other recommended therapies, should be used in patients with diabetes and CKD unless specific contraindications exist.
Nonacute Ischemic Syndromes. Optimal methods of coronary artery revascularization are controversial. Advances in this field are evolving so rapidly that technologies used in trials are often considered outdated by the time the results are published. Data specifically concerning people with diabetes and CKD are lacking, but for those with either diabetes or CKD, coronary artery bypass surgery has been considered superior to percutaneous transluminal angioplasty for multiple-vessel disease.38, 124, 125 The NKF-KDOQI™ Guidelines for CVD in Dialysis Patients came to a similar conclusion based on retrospective and observational data, while recommending research to include prospective controlled trials of newer stenting technologies.97 Much of the benefit of coronary artery bypass surgery in diabetes or CKD stage 5 appears to be derived from use of the internal mammary artery.38, 97
Studies of non–dialysis-dependent patients with CKD have been mostly observational cohort studies in which PCI did not consistently include stenting.124, 125 Since these studies were conducted, PCI approaches have progressed to almost routine use of coronary stents. In a recent subgroup analysis of a prospective clinical trial, the Arterial Revascularization Therapies Study, patients with a calculated creatinine clearance less than 60 mL/min/1.73m2 had similar survival free of death, myocardial infarction, or stroke whether they were randomly assigned to either coronary artery bypass surgery or PCI with multiple-vessel stenting.126 Only repeated revascularization was less frequent with coronary artery bypass surgery.
Most recently, drug-eluting stents containing sirolimus or paclitaxel were shown to largely prevent restenosis, the most common reason for long-term failure of bare metal stents.126-128 Although patients with diabetes have greater rates of restenosis and major adverse cardiac events after coronary artery stent placement, these complications were reduced markedly in the trials of drug-eluting stents.127-129 Addition of abciximab to stenting procedures in patients with diabetes also has been advocated to reduce restenosis, but has not demonstrated a benefit on clinical outcomes.130 Future studies using drug-eluting stents are likely to challenge the notion that coronary artery bypass surgery is the preferred method of revascularization in patients with diabetes.
Although controlled trials of revascularization procedures are nonexistent for people with both diabetes and CKD, the excess cardiovascular risk and deaths associated with diabetes after PCI were driven predominantly by the subset with proteinuria in a large observational cohort study.131 This group of patients should be included in clinical trials of innovative revascularization technologies in the future. In the meantime, the opinion of the Work Group is that either coronary artery bypass grafting or stenting (single or multiple vessel) appear to be acceptable methods of revascularization in people with diabetes and CKD. Decisions about revascularization procedures should be based on individual patient characteristics, local expertise, and best judgment of the treating physicians.
The Competing Risks Paradigm: CKD and CVD
People with diabetes and CKD are at high risk to both lose kidney function and experience major adverse cardiovascular events (Fig 4). Treatment of risk factors reduces the likelihood of these outcomes. Fortunately, treatment strategies for reducing kidney and cardiovascular risks are largely shared. The present CPGs and CPRs for diabetes and CKD are consistent with those already established for the treatment of diabetes and CVD by the ADA and AHA.34, 38 Goals of the management approaches recommended here are intended to mitigate the devastating consequences of the spectrum of vascular complications, including kidney, heart, and others.
New to the NKF-KDOQI™ Guidelines: Management of Hyperglycemia and General Diabetes Care in CKD
This is the first guideline in the NKF-KDOQI™ series to address management of hyperglycemia and general diabetes care in people with CKD. The purpose of Guideline 2 is to review the extensive literature regarding glycemic control and DKD, with an emphasis on benefits, as well as risks, of intensive treatment of blood glucose, and to provide recommendations for the care of people with diabetes complicated by kidney disease.
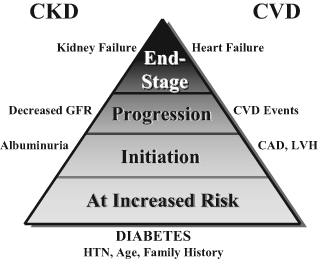
Figure 4. Diabetes Amplifies the CKD and CVD Paradigm.
Abbreviations: CAD, coronary artery disease; LVH, left ventricular hypertrophy; HTN, hypertension.
Hyperglycemia, the defining feature of diabetes, is a fundamental cause of vascular target-organ complications, including kidney disease. Intensive treatment of hyperglycemia prevents DKD and may slow progression of established kidney disease. An overall HbA1c goal of less than 7.0% for people with diabetes is supported by substantial data from large prospective randomized studies of both type 1 and type 2 diabetes. Much of this support stems from benefits for some of the other major complications of diabetes, especially retinopathy. With respect to kidney outcomes, data are very strong for the development of microalbuminuria (Guideline 2, Fig 8 to Fig 11).116, 132, 137 The numbers of patients progressing to more advanced outcomes, such as macroalbuminuria and low GFR, are decreased significantly with improved glycemic control, but much of this decrease is related to the smaller number developing microalbuminuria to begin with (Guideline 2, Fig 10 to Fig 12).116, 133-141 Nonetheless, even for those with more advanced disease, evidence supports reaching the recommended HbA1c target.
The ADA recommends an HbA1c level less than 7.0% or as close to normal as possible without excessive hypoglycemia.34 The major risk of attaining HbA1c levels less than 7.0% is the increasing development of hypoglycemia with lower glucose concentrations. For people with decreased kidney function (CKD stages 3 to 5), hypoglycemia is a major concern because of impaired clearance of insulin and some of the oral agents used to treat diabetes, as well as diminished kidney gluconeogenesis. The amount of gluconeogenesis is decreased with reduced kidney mass.142 Reduction in gluconeogenesis may reduce the ability of a patient who is becoming hypoglycemic as the result of excessive insulin/oral agent dosage or lack of food intake to defend against hypoglycemia. Although this effect is difficult to quantify, the kidney degrades about a third of the insulin, leading to a prolonged half-life when kidney function is reduced. Patients with type 1 diabetes receiving insulin who had significant serum creatinine level elevations (mean, 2.2 mg/dL) were reported to have a 5-fold increase in the frequency of severe hypoglycemia.143,144 Therefore, it is imperative that patients being treated intensively monitor their glucose levels closely and reduce doses of medicines (insulin and oral agents) as needed to avoid hypoglycemia (Guideline 2, Table 22 and Table 23).
A person with advanced CKD may no longer need to achieve good glycemic control to prevent deterioration in kidney function. However, intensive treatment of hyperglycemia may still prevent or slow the progression of retinopathy, neuropathy, and macrovascular disease. Survival improves with better glycemic control in patients on peritoneal dialysis145 and hemodialysis therapy.146 In the latter study, after adjustment for age and sex, HbA1c level was a significant predictor of survival (hazard ratio [HR], 1.13 per 1.0% increment of HbA1c; 95% CI, 1.03 to 1.25, P = 0.01).
Data for monitoring glycemic control in people with diabetes and CKD essentially are absent. Therefore, in the opinion of the Work Group, assessment of glycemic control in diabetes and CKD should follow the general standards recommended by the ADA.34 In people receiving multiple insulin injections, self-monitoring of blood glucose (SMBG) is recommended 3 or more times daily (before meals and at bedtime). In those receiving less frequent insulin injections, oral agents, or medical nutrition therapy alone, SMBG is useful in achieving glycemic goals. Postprandial SMBG testing also may be helpful, particularly in patients with gastroparesis, to achieve postprandial glucose goals. The optimal frequency of SMBG has not been established in patients with type 2 diabetes treated by oral agents, but the ADA recommends testing sufficiently often to reach glycemic goals (Guideline 2, Table 25). In addition, HbA1c level should be determined at least twice per year in stable patients who are achieving glycemic goals and more often, approximately every 3 months, in patients whose therapy has changed or who are not reaching goals.
The Work Group emphasizes prevention and treatment of all diabetic complications in people with diabetes and CKD. Assessment and management of CVD has been addressed in the preceding section. Management of retinopathy and foot care also is essential for optimal outcomes. In the absence of specific data in the diabetes and CKD population, the Work Group recommends following the standards set by the ADA.34 An ophthalmologist or optometrist who is experienced in the diagnosis and management of diabetic retinopathy should perform a comprehensive dilated-eye examination annually in all people with diabetes (Guideline 2, Table 26). Patients should be educated about the importance of foot surveillance and ulcer prevention with an emphasis on self-management, as discussed in CPR 4. The feet should be examined visually at each health care visit. A comprehensive foot examination, including visual inspection, Semmes-Weinstein monofilament testing, and use of a 128-Hz tuning fork for testing of vibratory sensation, should be performed annually. Because the risk of ulcers and amputations is increased in those with diabetes and CKD, referral to foot-care specialists for annual examinations and preventive care is encouraged.
Updates to the NKF-KDOQI™ Guidelines: Management of Hypertension, Dyslipidemia, and Nutrition
Previous guidelines from the NKF-KDOQI™ series have addressed hypertension, dyslipidemia, and nutrition in CKD.5,6,9 The purpose of Guidelines 3, 4, and 5 is to focus on care of people with both diabetes and CKD, summarize rapidly emerging literature in these fields, and translate the results into updated recommendations for clinicians.
Hypertension
The natural history of DKD is characterized by hypertension, increasing albuminuria, and decreasing GFR. In both types of diabetes, the natural history is similar, with the exception that onset of hypertension and vascular disease is earlier in the course of type 2 diabetes.147,148 Hypertension is one of the most common comorbidities in DKD (Guideline 3, Table 29). Because the studies cited in Guideline 3, Table 29, were published before the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), hypertension generally was defined as blood pressure greater than 140/90 mm Hg.149-153 The JNC 7 defines hypertension in those with diabetes or CKD as blood pressure greater than 130/80 mm Hg.154 Thus, the prevalence estimates in Guideline 3, Table 29, likely represent lower range values based on current criteria for hypertension in diabetes or CKD. A large number of epidemiological studies and controlled trials have defined hypertension as a risk factor for progression of DKD, and antihypertensive treatment reduces this risk (Guideline 3, Fig 18).5 Studies of people with type 1 or type 2 diabetes and CKD stages 1 to 4 were included in the evidence review. Based on the available evidence, the Work Group recommends a blood pressure target of less than 130/80 mm Hg with ACE inhibitors and ARBs as preferred agents, usually in combination with a diuretic, for the treatment of hypertension in diabetes and CKD (Guideline 3, Table 27). Because diabetes is highly prevalent, individuals with other types of CKD may have diabetes. The approach to antihypertensive treatment in DKD does not conflict with that recommended for CKD in general.34, 154
The emphasis of the evidence review was on the effects of treating hypertension on kidney outcomes, although control of blood pressure also is essential for reducing CVD risk. In people with either type 1 or type 2 diabetes and microalbuminuria, prevention of DKD progression by treatment with ACE inhibitors or ARBs is supported by moderate evidence.155-166 For the purpose of the current guidelines, this evidence was considered moderate rather than strong because of insufficient data for outcomes other than albuminuria (ie, decrease in GFR, CKD stage 5, or mortality). The Work Group seriously deliberated about whether progression of albuminuria is an acceptable surrogate outcome for progression of DKD. As detailed in CPR 1, they eventually concluded that further study of this issue is necessary to resolve the controversy. For those with hypertension and macroalbuminuria, evidence strongly supports use of ACE inhibitors in type 1 diabetes and ARBs in type 2 diabetes to prevent progression of DKD (Guideline 3, Fig 13 to Fig 15).167-169 In the view of the Work Group, the existing evidence has been influenced heavily by the design of the studies, which used ACE inhibitors in type 1 diabetes and ARBs in type 2 diabetes. Based on biological plausibility, similar modes of action, and smaller studies, the Work Group considers these 2 classes of agents essentially interchangeable and did not distinguish between them in the guideline statement.
To achieve target blood pressure, multiple antihypertensive agents usually are required (Guideline 3, Table 32). Therefore, most people with diabetes and CKD require medicines in addition to RAS inhibitors for optimal control of hypertension. Diuretics are especially useful in this population. β-Blockers and calcium channel blockers also are effective therapies. Based on a series of small studies and the Irbesartan Diabetic Nephropathy Trial (IDNT), calcium channel blockers of the dihydropyridine class may worsen proteinuria and failed to improve clinical outcomes when used as primary antihypertensive therapy in DKD.170,171 Conversely, in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) subgroup with type 2 diabetes and CKD (defined as GFR < 60 mL/min/1.73 m2), amlodipine was comparable to lisinopril or chlorthalidone for GFR decrease or onset of kidney failure when each agent was given separately.172 However, the lack of albuminuria/proteinuria data and relatively limited sample size in this substudy preclude firm conclusions. Based on numerous studies of proteinuric kidney diseases (DKD and non-DKD),154 it was the opinion of the Work Group that dihydropyridine calcium channel blockers should not be used in the absence of concurrent RAS inhibition for DKD characterized by microalbuminuria or macroalbuminuria. However, dihydropyridine calcium channel blockers appear to be safe in such patients if they also use an ACE inhibitor or an ARB.173
Dyslipidemia
Dyslipidemia is common in people with diabetes and CKD. Modifying CVD risk by using lipid-lowering agents is of great importance, as discussed (in Diabetes, CKD, and CVD). The NKF-KDOQI™ CPGs for Managing Dyslipidemia in CKD Patients were published recently,6 and the CPGs for CVD in Dialysis Patients added new information about the management of dyslipidemia in dialysis patients.10 Guideline 4 focuses specifically on patients with diabetes and CKD stages 1 to 5. In general, the guidelines for use of lipid-lowering agents in CKD stages 1 to 4 due to diabetes and other causes do not conflict,174-177 although there is no direct or indirect evidence for treating patients with CKD stage 4. The Work Group recommends that people with diabetes and CKD stages 1 to 4 be treated according to current guidelines for groups at high CVD risk.6, 175 Therefore, the target low-density lipoprotein cholesterol (LDL-C) level should be less than 100 mg/dL, with less than 70 mg/dL as a therapeutic option (Guideline 4, Table 36). Lipid-lowering agents in the statin class are the preferred drug therapies. However, treatment with a statin should not be initiated in patients with type 2 diabetes on maintenance hemodialysis therapy who do not have a specific cardiovascular indication for treatment because of negative results for CVD outcomes reported recently in the 4D (Fig 5).100 This finding represents an update from previous guidelines because 4D was the first prospective randomized trial in hemodialysis patients with diabetes.6,10, 100 Indirect evidence on the beneficial effects of pravastatin in diabetes and CKD stages 1 to 3 from recent post hoc analyses of large multicenter trials also was added.99 Recommendations for treatment of dyslipidemia in diabetes and CKD are based on CVD risk reduction because the current state of evidence is insufficient to support treatment for preservation of kidney function. In the opinion of the Work Group, studies to determine effects of statins or other lipid-lowering agents on progression of kidney disease are critically important to the goal of optimizing care for people with diabetes and CKD.
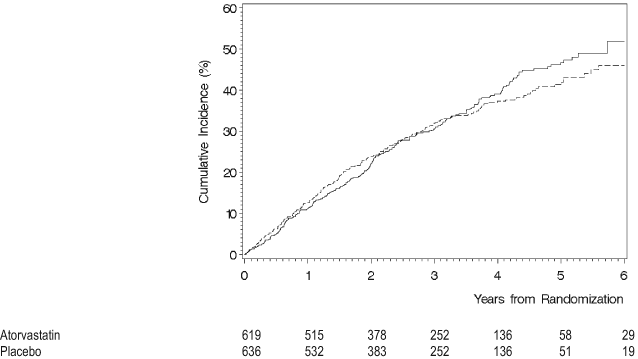
Figure 5. Cumulative Incidence Estimate of the Combined Primary End Point for Placebo and Atorvastatin Treatment Groups in the 4D.
Solid line: placebo; dotted line: atorvastatin treatment. Reprinted with permission.100
Nutrition
Management of diabetes and CKD should include nutritional intervention. Guideline 5 addresses dietary strategies in people with diabetes and CKD stages 1 to 4. Dietary recommendations for CKD stage 5 are provided in the KDOQI™ CPGs for Nutrition in Chronic Renal Failure.9 Nutritional management for people with diabetes has focused traditionally on blood glucose control. However, dietary modifications may reduce the progression of CKD, as well. In particular, dietary protein intake at all stages of CKD appears to have an important impact in the population with diabetes. When dietary protein is limited, adequate caloric intake must be maintained by increasing calories from carbohydrates and/or fats. Competing needs for nutritional management of hyperglycemia, hypertension, and dyslipidemia can make determination of appropriate protein intake challenging.
A dietary protein intake of 0.8 g/kg body weight per day (about 10% of total calories), the recommended daily allowance (RDA) for this macronutrient, is a level that has been achieved in studies of nutritional intervention for diabetes and CKD. Nutrition surveys indicate that most Americans eat in excess of the RDA level.178 In 2 meta-analyses, low-protein diets reduced risks of progression of albuminuria/proteinuria and loss of GFR, with more pronounced benefits in DKD than non-DKD (Guideline 5, Fig 21).179,180
More recently, even a modest limitation of dietary protein (0.89 versus 1.02 g/kg body weight per day) reduced the risk of CKD stage 5 or death (relative risk [RR], 0.23; 95% CI, 0.07 to 0.72; P = 0.04) in people with type 1 diabetes and stage 2 CKD (inferred based on levels of albuminuria and GFR; Guideline 5, Fig 22).181 Benefits of limiting dietary protein intake are more evident in type 1 than type 2 diabetes, but fewer studies have been done in the latter population. Based on the available evidence, the Work Group concluded that limiting dietary protein to the RDA level of 0.8 g/kg body weight per day should stabilize or reduce albuminuria, slow the decrease in GFR, and may prevent CKD stage 5.179-186 The current recommendation for dietary protein in diabetes and CKD stages 1 to 4 represents an update to the diet recommended by the NKF-KDOQI™ CPGs for Hypertension and Antihypertensive Agents in CKD (Guideline 5, Table 43).5
At the other end of the spectrum, high-protein diets are a special concern in patients with diabetes because they may increase albuminuria and accelerate loss of kidney function. Based on both studies of humans and experimental models, higher protein intake appears to produce more profound glomerular hyperfiltration and kidney damage in diabetes.153, 187-196 Emerging epidemiological evidence indicates that higher protein intake (≥20% versus 10% of total daily calories) is associated with loss of kidney function in women with mildly decreased GFR (CKD stages 1 to 2 inferred) and the development of microalbuminuria in people with diabetes and hypertension.197,198 Therefore, in the opinion of the Work Group, people with diabetes and CKD should avoid high-protein diets (≥20% of total daily calories). Some common fad diets that recommend high protein are Atkins® Protein Power, the Zone, South Beach®, and Sugar Busters®.
In the Dietary Approaches to Stop Hypertension (DASH) and DASH-Sodium diets, a relatively high protein intake (1.4 g/kg body weight per day, or about 18% of total calories) is recommended.199 Sources of protein in the DASH diets emphasize vegetables, low-fat or nonfat dairy products, whole grains, nuts, legumes, fish, and poultry. Red meat is eaten in only small amounts. In recent studies of people with prehypertension or untreated stage 1 hypertension, higher protein intake from either soy or predominantly vegetable sources decreased blood pressure in short-term (6 to 12 weeks) feeding studies.200,201 Along with the DASH trials, these data suggest that predominantly nonmeat protein may have a beneficial effect on blood pressure. Small studies suggest that vegetable or soy protein sources may be kidney sparing compared with red meat sources in diabetes and CKD.182, 202 Furthermore, the risk of losing kidney function in women with mildly decreased GFR in the Nurses Health Study was related primarily to animal meat intake.197,198 Therefore, a DASH-type diet that emphasizes sources of protein other than red meat may be a reasonable alternative to a lower total protein intake in people with hypertension, diabetes, and CKD stages 1 to 2.
New to NKF-KDOQI™ CPRs:
How Should Albuminuria Be Managed in Normotensive Patients With Diabetes?
Increased levels of urinary albumin excretion predict increased risk of kidney and CVD outcomes in diabetes, as reviewed extensively in Guideline 1 and the preceding section, Diabetes, CKD, and CVD. Albuminuria is believed to reflect endothelial injury that extends from the glomerulus to the arterial circulation at large, thus linking this marker to both kidney disease and CVD. The concept that treatments aimed at decreasing albuminuria may improve clinical outcomes has been a subject of great interest and debate.
CPR 1 addresses the evidence for treatment of normotensive patients who have diabetes and elevated albuminuria with RAS inhibitors. Relatively few studies of these antihypertensive agents have recruited normotensive patients. In a study of type 1 diabetes with macroalbuminuria, ACE inhibitors decreased albuminuria and reduced the risk of clinical outcomes (doubling of serum creatinine level, CKD stage 5, or death) regardless of the presence or absence of hypertension.168 A quarter of the participants in this study were normotensive. There was no significant difference in the treatment effect between normotensive and hypertensive individuals. In type 2 diabetes with macroalbuminuria, ARB treatment also reduced the risk of clinical outcomes in 2 separate studies.167, 169 However, these studies had very few participants with normal blood pressure. Treatment of microalbuminuria by ACE inhibition in normotensive people with type 1 diabetes reduces the level of albuminuria and prevent progression to macroalbuminuria in a meta-analysis.203 A small study of normotensive patients with type 1 diabetes showed that ACE inhibition prevented new-onset microalbuminuria.204 Several studies have evaluated ACE inhibition in normotensive people with type 2 diabetes and microalbuminuria.104, 205-207 All studies demonstrated decreased progression to macroalbuminuria and/or reduced levels of albuminuria.
In the opinion of the Work Group, change in level of albuminuria or transition between categories (normoalbuminuria, microalbuminuria, or macroalbuminuria) in normotensive people with diabetes is relatively weak evidence for change in status or prognosis of kidney disease. The rationale for this opinion is as follows. First, level of albuminuria or crossing an albumin-creatinine ratio (ACR) threshold is not a clinical end point. Second, RAS inhibitors might mask the progression of DKD marked by albuminuria. In type 1 diabetes, withdrawal of ACE inhibition caused a rapid increase in albuminuria,208 and in type 2 diabetes, discontinuation of irbesartan in the Irbesartan in Patients With Type 2 Diabetes and Microalbuminuria (IRMA-2) Study prompted a rapid return to pretreatment levels of albuminuria in patients receiving the lower dose of irbesartan and a partial return to pretreatment levels in those receiving the higher dose of irbesartan.209 Third, few normotensive patients with diabetes and microalbuminuria or macroalbuminuria have been enrolled in clinical trials of treatments for kidney disease. The demonstrated benefits of RAS inhibitors for reducing and stabilizing albuminuria were noted; however, in the absence of studies with clinical end points, the Work Group found this evidence insufficient to justify a higher evidence rating.
Despite these concerns, the consensus of the Work Group was that the benefit of ACE inhibitors and ARBs for reducing albuminuria and delaying kidney disease progression are likely to be similar among most people with diabetes and microalbuminuria or macroalbuminuria regardless of their blood pressure level. Therefore, CPR 1 recommends treatment with RAS inhibition for normotensive patients with diabetes and microalbuminuria or macroalbuminuria. The Work Group encourages further research to determine effects of ACE inhibitors and ARBs on albuminuria and clinical outcomes in normotensive people with DKD.
Is Albuminuria an Acceptable Surrogate Marker for Progression of DKD?
CPR 1 addresses whether changes in albuminuria are sufficient to predict clinical outcomes in DKD. Studies testing the hypothesis that albuminuria reduction predicts improved prognosis in DKD have been performed only as secondary analyses of studies of ARB treatment in people with type 2 diabetes and macroalbuminuria.210-212 In these studies, level of albuminuria reduction was a marker of decreased risk of adverse outcomes. Observational analyses from the Reduction of Endpoints in Non–insulin-dependent diabetes mellitus (NIDDM) with the Angiotensin II Antagonist Losartan (RENAAL) trial found that the magnitude of albuminuria reduction predicted reduced risk for both CVD events and kidney end points (CPR 1, Fig 23 and Fig 24).211,212 Similarly, an analysis from the IDNT found that degree of proteinuria reduction corresponded to decreased kidney end points (CPR 1, Fig 25).210 These findings raise the hypothesis that albuminuria reduction per se has beneficial effects. However, an alternative possibility is that albuminuria reduction is a marker for patients with less severe kidney and vascular disease. A strategy of targeting treatment of albuminuria, in addition to blood pressure and other risk factors, has not been tested prospectively in patients with diabetes. Furthermore, to date, only these secondary analyses from the RENAAL trial and IDNT have directly correlated albuminuria/proteinuria reduction with clinical benefit.
In the opinion of the Work Group, there currently is insufficient evidence to assume that lowering albuminuria levels will necessarily lead to improvements in such clinical outcomes as progression to CKD stage 5, CVD events, or death. Conversely, the failure to reduce albuminuria does not preclude a beneficial clinical effect on DKD from a potential intervention. Therefore, to be considered efficacious, potential treatments for DKD must demonstrate benefits not only on albuminuria reduction, but also on such clinical end points as CKD stage 5, CVD events, or death.213 Nevertheless, the emerging data generate a strong hypothesis that should be tested in prospective controlled studies—namely, do treatments (ACE inhibitors, ARBs, or others) that decrease albuminuria result in improved CKD and CVD outcomes in people with diabetes?
The Value of Multifaceted Intervention
Although these and other guidelines present recommendations for management of risk factors separately, in reality, multiple risk factors are managed concurrently in patients with diabetes and CKD. In addition, considering the burgeoning epidemic of obesity and its role in producing diabetes and, possibly, kidney disease, the importance of weight control should be considered in the care of patients with diabetes and CKD. CPR 2 was developed to address these issues and encourage further investigation.
In the Steno Study, a multifaceted approach aimed at optimal management for a group of risk factors was evaluated in patients with type 2 diabetes and microalbuminuria.45,46 The intervention had multiple targets, including behavioral modification and pharmacological therapies for hyperglycemia, hypertension (emphasizing RAS inhibitors), dyslipidemia, CVD prevention with aspirin, and a vitamin/mineral supplement (CPR 2, Table 1). This intensive intervention was compared with usual care. A mean decrease in albuminuria (albumin decreased 20 mg/24 h) was observed in the intensive-intervention group, whereas a mean increase occurred in patients in the usual-care group (albumin increased 30 mg/24 h). Albuminuria progression and the composite outcome of CVD events or death were decreased in the group treated intensively (CPR 2, Fig 26). However, which facets of the intervention are associated with reduced risk is uncertain. Furthermore, because the intensive intervention increased use of RAS inhibitors, the contribution of other treatments is unclear. Despite these limitations, the Work Group recognizes the importance of addressing multiple risk factors in an integrated fashion. The incremental effects of a multifaceted approach appear to add up to substantial clinical benefits.
Obesity now is recognized as a risk factor for diabetes, hypertension, CVD, and possibly CKD. Recent estimates from NHANES report that 31% of the US population is obese (BMI > 30 kg/m2).214 A growing body of evidence indicates that obesity is linked to CKD.215-221 Whether this link is independent of diabetes, hypertension, or perhaps other risk factors is not yet clear. Nevertheless, obesity is associated with the development of proteinuria and loss of kidney function. Metabolic syndrome risk factors, as well as adipose-derived factors, may lead to kidney damage. Maintaining a normal weight (BMI, 18.5 to 24.9 kg/m2) improves risk factors and may decrease the development or progression of CKD. The Work Group recommends that weight loss be achieved by a balanced reduction in caloric intake, rather than by diets that derive excess calories (>20%) from animal protein (Guideline 2). Regular physical exercise also is encouraged to assist in achieving and maintaining a normal weight.
Lifestyle and Behavioral Management
Strategies for behavioral change and self-management of risk factors are addressed in CPR 4. Because of the paucity of data in the diabetes and CKD population, these recommendations were extrapolated from data in other groups and thus are included in the CPR section. A proposed approach to a diabetes and CKD self-management program is provided in CPR 4, Table 56.
At the core of the diabetes epidemic and its consequent complications is a fundamental shift in lifestyle. In a relatively short time span, vigorous physical activity and limited calories have been replaced by sedentary behavior and a seemingly endless array of calorie-dense foods that are cheap and easily obtained. Thus, major challenges of the present century are the dual problems of overfeeding and obesity. Optimal management of risk factors, including hypertension, diabetes, and dyslipidemia, is emphasized inthese guidelines. However, this emphasis is directed too often toward drug therapies without enough attention to key lifestyle issues. In the view of the Work Group, addressing lifestyle through behavioral change is critically important for success in reducing the devastating impact of diabetes and CKD. The Work Group considers investigation in this area of particular importance to successfully translate advances in knowledge to improvements in quality of life and health.