Chronic kidney disease (CKD) is a worldwide public health problem affecting more than 50 million people, and more than 1 million of them are receiving kidney replacement therapy.1,2 The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative™ (NKF-KDOQI™) Clinical Practice Guidelines (CPGs) and Clinical Practice Recommendations (CPRs) on CKD estimates that CKD affects 11% of the US population,3 and those affected are at increased risk of cardiovascular disease (CVD) and kidney failure. Kidney failure represents about 1% of the prevalent cases of CKD in the United States,3 and the prevalence of kidney failure treated by dialysis or transplantation is projected to increase from 453,000 in 2003 to 651,000 in 2010.3,4
Management of CKD is costly. The Medicare CKD stage 5 population nearly doubled in the last 10 years, and the CKD population expanded, as well. Together, they account for 16.5% of Medicare expenditures, nearly double that of 10 years ago, and the total costs for kidney disease now approach 24% of Medicare expenditures.4 A growing body of evidence suggests that some of the adverse outcomes of CKD can be prevented or delayed by preventive measures, early detection, and treatment.
NKF-KDOQI™ CPGs presently offer strategies to manage hypertension,5 dyslipidemia,6 bone disease,7 anemia,8 nutrition,9 and CVD10 in patients with CKD. The present Guidelines extend the scope of the NKF-KDOQI™ CPGs and CPRs by offering strategies to diagnose and manage patients with diabetes and CKD.
Epidemic of Diabetes
Nearly 21 million people in the United States, or 7% of the population, have diabetes, and about a third of those with diabetes are unaware they have the disease. About 5% to 10% of diabetes in the United States is type 1, which develops as a consequence of the body's failure to produce insulin. In some racial and ethnic groups, the proportion of cases attributable to type 1 diabetes is even less.11 Most cases of diabetes in the United States and elsewhere are type 2, which develops because of the body's failure to produce sufficient insulin and properly use the insulin it produces. Worldwide, 171 million people have diabetes.
Diabetes prevalence is increasing most rapidly in the developed countries and in developing countries undergoing transition from traditional to modern lifestyles.12,13 In the general US population, estimates from national surveys14 show an 8-fold increase in the prevalence of diagnosed diabetes between 1958 and 2000. The San Antonio Heart Study15 suggests an increasing incidence rate of type 2 diabetes is responsible, in part, for the increasing prevalence among Mexican Americans and for a borderline significant trend in non-Hispanic whites. The investigators attribute the greater prevalence of diabetes in this population more to the increasing incidence than to the decrease in cardiovascular mortality reported among people with diabetes nationally.16 Other factors responsible for the increasing prevalence of diabetes include changes in diagnostic criteria, increased public awareness, decreasing overall mortality, growth in minority populations, a dramatic increase in the magnitude and frequency of obesity, and the widespread adoption of a sedentary lifestyle.14 Most of the increase in diabetes prevalence is attributable to type 2 diabetes, and although much of this increase is occurring in adults, children and adolescents increasingly are affected. However, a worldwide increase in the incidence of type 1 diabetes also has been noted, particularly among children younger than 5 years.17
Projections of the future burden of diabetes in the US population suggest that the prevalence of diabetes will increase 165% between 2000 and 2050, with the greatest increases in the population older than 75 years and among African Americans.18 The global burden of diabetes is expected to double between 2000 and 2030, with the greatest increases in prevalence occurring in the Middle East, sub-Saharan Africa, and India.19 Moreover, the development of type 2 diabetes during the childbearing years also will increase, primarily in the developing countries (CPR 3, Fig 27).19 Projections regarding the future burden of diabetes are based on increasing life expectancy, population growth, and progressive urbanization.20 Of growing concern is the belief that these estimates may be too low because they do not account for the increasing frequency and magnitude of obesity and other major risk factors for diabetes.
As the population of patients with diabetes of long duration grows, reports of a dramatically increasing burden of diabetic kidney disease (DKD) are appearing from developed countries,21 as well as from Africa,22,23 India,24 the Pacific Islands,25 and Asia,26,27 where infectious disease previously posed the greatest threat28 (see CPR 3). Increased risk and more rapid progression of DKD29,30 also have been reported in immigrants from developing to developed countries.31,32
Diabetes is the leading cause of CKD in developed countries and rapidly is becoming the leading cause in developing countries as a consequence of the global increase in type 2 diabetes and obesity.33 In the United States, microalbuminuria is found in 43%, and macroalbuminuria, in 8% of those with a history of diabetes.3 Moreover, diabetes accounts for 45% of prevalent kidney failure, up from 18% in 1980.4
Substantial underdiagnosis of both diabetes and CKD leads to lost opportunities for prevention, and inadequate or inappropriate care of patients with diabetes and CKD may contribute to disease progression. Nevertheless, diabetes care has improved as the benefits of meticulous management have become widely accepted and the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins has increased in patients with diabetes.4 Even so, fewer than 1 in 4 patients with diabetes receives at least 1 hemoglobin A1c(HbA1c) test, at least 1 lipid test, and at least 1 glucose testing strip each year, reflecting the need for better assessment of these high-risk patients.4
DKD refers to kidney disease that is specific to diabetes. Although kidney biopsy is required to diagnose diabetic glomerulopathy definitively, in most cases, careful screening of diabetic patients can identify people with DKD without the need for kidney biopsy. DKD is based in part on the finding of elevated urinary albumin excretion, which is divided arbitrarily into: (1) microalbuminuria, a modest elevation of albumin thought to be associated with stable kidney function, but a greater risk of macroalbuminuria and kidney failure; and (2) macroalbuminuria, a higher elevation of albumin associated with progressive decline in glomerular filtration rate (GFR), an increase in systemic blood pressure, and a high risk of kidney failure.
Most professional societies concerned with diabetes and kidney disease now advocate screening for microalbuminuria in patients with diabetes, and the suggested screening plan, adapted from the American Diabetes Association (ADA) guideline, is shown in Guideline 1, Fig 6.34,35 Screening should begin 5 years after diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes because of the inability to establish the onset of type 2 diabetes with certainty. Because urinary albumin excretion has an intraindividual coefficient of variation (CV) of approximately 40%,36 multiple positive test results are required for classification. Definitions of DKD by albuminuria and stage are shown in Guideline 1, Table 6.
Evidence for the usefulness of estimated GFR (eGFR) alone as a screening test for CKD in diabetes is less secure. Many patients with diabetes and CKD may have elevated or high-normal GFRs, particularly in the early years after diagnosis. Therefore, markers of kidney damage are required to detect early stages of CKD; eGFR alone can only detect CKD stage 3 or worse (Guideline 1, Table 6).
Because diabetes is a common condition, coincidence with other nondiabetic CKD is relatively frequent. Accordingly, evaluation of a person with atypical features should, in selected cases, include additional diagnostic testing, depending on the clinical presentation. Care should be used in determining the appropriate diagnostic tests because administration of radiographic contrast, with or without angiography, may pose greater risks in people with diabetes and CKD than in others.
Diabetes is one of the most important risk factors for CVD. The risk imparted by diabetes has been described as a CVD risk equivalent because the likelihood of future events may approach that of people without diabetes who have already had a myocardial infarction.37 Such observations have led to recommendations from both the ADA and the American Heart Association (AHA) for intensive cardiovascular risk factor management in people with diabetes (Table 1).34,38 CKD also imparts an extremely high risk of CVD. The NKF and the AHA recently issued guidelines and scientific statements recommending that people with CKD be considered in the highest risk category for CVD.3,39 For those with both diabetes and CKD, the outlook is far worse than for either condition alone because this combination is a powerful predictor of major adverse cardiovascular events and death. The relationship between CKD severity and risk is continuous. People with diabetes and microalbuminuria have twice the CVD risk of those with normoalbuminuria,40 and as albuminuria increases and GFR decreases, CVD risk increases progressively.41,43 In an analysis of patients with type 2 diabetes from the UK Prospective Diabetes Study (UKPDS), rates of death and progression to macroalbuminuria were equal at the microalbuminuric stage.41 However, at the macroalbuminuric stage, the death rate outpaced the rate of kidney disease progression (Fig 2). More people who reach CKD stage 3 will die, primarily of CVD, than progress to kidney failure, especially if they also have diabetes.3,44
In the Background, a focused review of relationships among diabetes, CKD, and CVD relevant to people with CKD stages 1 to 4 is presented. The review includes a discussion of intensive risk factor management for the prevention of CVD, the evaluation of coronary heart disease in patients with diabetes, and medical management and coronary revascularization in these patients. Specific recommendations for CKD stage 5 are provided in the NKF-KDOQI™ Guidelines for CVD in Dialysis Patients.10
People with diabetes and CKD are at high risk to both lose kidney function and experience major adverse cardiovascular events (Background, Fig 4). Treatment of risk factors reduces the likelihood of these outcomes. Fortunately, treatment strategies are largely shared for reducing kidney and cardiovascular risks. The present CPGs and CPRs for diabetes and CKD are consistent with those already established for the treatment of diabetes and CVD by the ADA and AHA.34,38 Goals of the management approaches recommended here are intended to mitigate the devastating consequences of the spectrum of vascular complications, including kidney, heart, and others.
These CPGs seek to improve outcomes in patients with diabetes and CKD by providing strategies for the diagnosis (Guideline 1) and management (Guidelines 3 to 5 and CPRs 1 to 4) of CKD in the setting of diabetes and for the management of diabetes in the setting of CKD (Guideline 2). The general treatment of diabetes is beyond the scope of this guideline and is addressed comprehensively in the ADA guidelines.34
As part of an evolution in the development of CPGs, the Work Group divided its recommendations, which are based on a systematic review of the literature, into a series of Guidelines and CPRs. The Guidelines were based on a consensus within the Work Group that the strength of the evidence was sufficient to make definitive statements about appropriate clinical practice. When the strength of the evidence was not sufficient to make such statements, the Work Group offered CPRs based on the best available evidence and expert opinion. As new data become available, the strength of the evidence for many of the CPRs may become sufficient for the CPRs to become CPGs, illustrating the need for recurring reviews and updates of this document. Many of the research recommendations proposed by the Work Group were developed with the goal of strengthening the evidence for the CPRs to determine whether they should become Guidelines in the future.
The term “definitive” must be used with caution, particularly in the context of CPGs. Uncertainty is an immutable element of all scientific research, and the establishment of a Guideline should neither preclude nor render unethical further inquiry. Rather, the establishment of guidelines represents an evolving process that seeks to ensure that each patient receives the best possible care within the context of presently available medical knowledge.

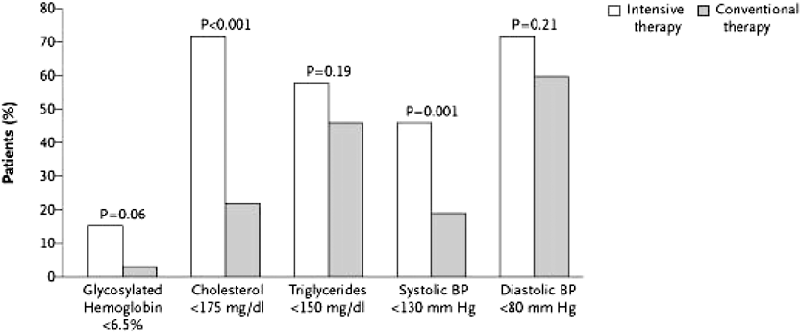
Figure 1. Percentage of Patients in Each Group of the Steno Study Who Reached the Intensive-Treatment Goals at a Mean of 7.8 Years.
Abbreviation: BP, blood pressure. Reprinted with permission.45
Scope
The target population of these CPGs is patients with CKD stages 1 to 5, including dialysis and transplant patients. However, the emphasis is on stages 1 to 4 because the evidence in stage 5 is either lacking or addressed in other NKF-KDOQI™ Guidelines. Consideration is given to the diagnosis, impact, and management of diabetes and CKD in children, adults, the elderly, pregnant women, and different racial and ethnic groups.
The intended readers are practitioners who manage patients with diabetes and CKD, including, but not limited to, primary care providers, nephrologists, cardiologists, endocrinologists/diabetologists, physician’s assistants, nurse practitioners, nurses, dietitians, pharmacists, social workers, and diabetes educators. By reviewing scientific evidence from throughout the world, coordinating our efforts with guideline development processes elsewhere, and including in the Work Group experts from Latin America and Europe, as well as from North America, we believe this document has relevance beyond practitioners in North America.
The Value of Multifaceted Intervention
Although these and other guidelines present recommendations for the management of risk factors separately, in reality, multiple risk factors are managed concurrently in patients with diabetes and CKD. In the Steno Study, a multifaceted approach aimed at optimal management for a group of risk factors was evaluated in patients with type 2 diabetes and microalbuminuria.45,46 The intervention had multiple targets, including behavioral modification and pharmacological therapies for hyperglycemia, hypertension (emphasizing renin-angiotensin system [RAS] inhibitors), dyslipidemia, CVD prevention with aspirin, and a vitamin/mineral supplement (CPR 2, Table 48). This intensive intervention was compared with usual care. A mean decrease in albuminuria (albumin decreased 20 mg/24 h) was observed in the intensive-intervention group, whereas a mean increase occurred in patients in the usual-care group (albumin increased 30 mg/24 h). Albuminuria progression and the composite outcome of CVD events or death were decreased in the group treated intensively (CPR 2, Fig 26). However, which facets of the intervention are associated with reduced risk is uncertain. Furthermore, because the intensive intervention increased the use of RAS inhibitors, the contribution of other treatments is unclear. Despite these limitations, the Work Group recognizes the importance of addressing multiple risk factors in an integrated fashion. The incremental effects of a multifaceted approach appear to add up to substantial clinical benefits, even when each of the therapeutic goals is not met (Fig 1). A long-term, targeted, intensive intervention involving multiple risk factors and using currently available therapeutic agents reduces the risk of cardiovascular and microvascular events by about 50% among patients with type 2 diabetes and microalbuminuria.45
Multiple important, but unanswerable, questions arose during the development of each Guideline and CPR. These questions led to research recommendations that should be high priorities to improve the care of patients with diabetes and CKD. The Work Group recognizes the importance of bringing new treatments into clinical research for DKD, especially for patients who have progressive kidney disease despite the current standard of care. Promising treatments, including novel agents and potential new uses of existing agents, are currently in phase 2/3 trials for DKD. These recommendations and the new treatments in clinical trials are described in the Research Recommendations section.
Guideline 1: Screening and Diagnosis of DKD
CKD in patients with diabetes may or may not represent DKD. In the absence of an established diagnosis, the evaluation of patients with diabetes and kidney disease should include investigation into the underlying cause(s).
1.1 Patients with diabetes should be screened annually for DKD. Initial screening should commence:
- 5 years after the diagnosis of type 1 diabetes; (A) or
- From diagnosis of type 2 diabetes. (B)
1.1.1 Screening should include:
- Measurements of urinary albumin-creatinine ratio (ACR) in a spot urine sample; (B)
- Measurement of serum creatinine and estimation of GFR. (B)
1.2 An elevated ACR should be confirmed in the absence of urinary tract infection with 2 additional first-void specimens collected over the next 3 to 6 months. (B)
- Microalbuminuria is defined as an ACR between 30-300 mg/g.
- Macroalbuminuria is defined as an ACR > 300 mg/g.
- 2 of 3 samples should fall within the microalbuminuric or macroalbuminuric range to confirm classification.
1.3 In most patients with diabetes, CKD should be attributable to diabetes if:
- Macroalbuminuria is present; (B) or
- Microalbuminuria is present
- in the presence of diabetic retinopathy, (B)
- in type 1 diabetes of at least 10 years' duration. (A)
1.4 Other cause(s) of CKD should be considered in the presence of any of the following circumstances: (B)
- Absence of diabetic retinopathy;
- Low or rapidly decreasing GFR;
- Rapidly increasing proteinuria or nephrotic syndrome;
- Refractory hypertension;
- Presence of active urinary sediment;
- Signs or symptoms of other systemic disease; or
- >30% reduction in GFR within 2-3 months after initiation of an ACE inhibitor or ARB.
Guideline 2: Management of Hyperglycemia and General Diabetes Care in CKD
Hyperglycemia, the defining feature of diabetes, is a fundamental cause of vascular target-organ complications, including kidney disease. Intensive treatment of hyperglycemia prevents DKD and may slow progression of established kidney disease.
2.1 Target HbA1c for people with diabetes should be < 7.0%, irrespective of the presence or absence of CKD. (A)
Guideline 3: Management of Hypertension in Diabetes and CKD
Most people with diabetes and CKD have hypertension. Treatment of hypertension slows the progression of CKD.
3.1 Hypertensive people with diabetes and CKD stages 1-4 should be treated with an ACE inhibitor or an ARB, usually in combination with a diuretic. (A)
3.2 Target blood pressure in diabetes and CKD stages 1-4 should be < 130/80 mm Hg. (B)
Guideline 4: Management of Dyslipidemia in Diabetes and CKD
Dyslipidemia is common in people with diabetes and CKD. The risk of CVD is greatly increased in this population. People with diabetes and CKD should be treated according to current guidelines for high-risk groups.
4.1 Target low-density lipoprotein cholesterol (LDL-C) in people with diabetes and CKD stages 1-4 should be < 100 mg/dL; <70 mg/dL is a therapeutic option. (B)
4.2 People with diabetes, CKD stages 1-4, and LDL-C ≥ 100 mg/dL should be treated with a statin. (B)
4.3 Treatment with a statin should not be initiated in patients with type 2 diabetes on maintenance hemodialysis therapy who do not have a specific cardiovascular indication for treatment. (A)
Guideline 5: Nutritional Management in Diabetes and CKD
Management of diabetes and CKD should include nutritional intervention. Dietary modifications may reduce the progression of CKD.
5.1 Target dietary protein intake for people with diabetes and CKD stages 1-4 should be the recommended daily allowance (RDA) of 0.8 g/kg body weight per day. (B)
CPR 1: Management of Albuminuria in Normotensive Patients With Diabetes and Albuminuria as a Surrogate Marker
Treatments that decrease urinary albumin excretion may slow the progression of DKD and improve clinical outcomes, even in the absence of hypertension. However, most people with diabetes and albuminuria have hypertension; management of hypertension in these patients is reviewed in Guideline 3.
1.1 Normotensive people with diabetes and macroalbuminuria should be treated with an ACE inhibitor or an ARB. (C)
1.2 Treatment with an ACE inhibitor or an ARB may be considered in normotensive people with diabetes and microalbuminuria. (C)
1.3 Albuminuria reduction may be considered a treatment target in DKD. (C)
CPR 2: Multifaceted Approach to Intervention in Diabetes and CKD
Multiple risk factors are managed concurrently in patients with diabetes and CKD, and the incremental effects of treating each of these risk factors appear to add up to substantial clinical benefits.
2.1 The care of people with diabetes and CKD should incorporate a multifaceted approach to intervention that includes instruction in healthy behaviors and treatments to reduce risk factors. (C)
2.2 Target body mass index (BMI) for people with diabetes and CKD should be within the normal range (18.5-24.9 kg/m2). (C)
CPR 3: Diabetes and CKD in Special Populations
The increasing incidence of diabetes in children, young adults, the elderly, and members of disadvantaged and transitional populations is responsible for an increasing incidence of DKD in these groups. Racial/ethnic differences in susceptibility to DKD also may play a role. In pregnant women, the presence of diabetes and CKD may adversely affect the health of both the mother and her offspring.
3.1 Screening and interventions for diabetes and CKD should focus on populations at greatest risk. (C)
3.2 Although management of diabetes and CKD in special populations should follow the same principles as management in the majority population, there are special considerations in the treatment of children, adolescents, and the elderly. (C)
3.3 Population-based interventions may be the most cost-effective means for addressing the burden of CKD in special populations. Implementation and evaluation of population-based interventions should take into account the heterogeneity of the populations at risk. (C)
3.4 Specialists in high-risk pregnancy and kidney disease should co-manage pregnancy in women with diabetes and CKD. (C)
3.5 Treatment of DKD with RAS inhibitors before pregnancy may improve fetal and maternal outcomes, but these medicines should be discontinued as soon as a menstrual period is missed or after a positive pregnancy test. (C)
3.6 Insulin should be used to control hyperglycemia if pharmacological therapy is necessary in pregnant women with diabetes and CKD. (C)
CPR 4: Behavioral Self-Management in Diabetes and CKD
Behavioral self-management in patients with diabetes and CKD is particularly challenging because of the intensive nature of the diabetes regimen. Education alone is not sufficient to promote and sustain healthy behavior change, particularly with such a complex regimen.
4.1 Self-management strategies should be key components of a multifaceted treatment plan with attention to multiple behaviors: (C)
- Monitoring and treatment of glycemia,
- Blood pressure,
- Nutrition,
- Smoking cessation,
- Exercise, and
- Adherence to medicines.