4.1 High-Flux Membrane:
When methods to achieve good dialysate water quality are available, high-flux HD membranes should be used, defined as those providing β2-microglobulin (β2M) clearance of at least 20 mL/min under conditions of actual use.4.2 Minimum dose with hemofiltration or hemodiafiltration:
The recommended minimum delivered dose target, measured by using pretreatment and posttreatment BUN levels, is the same as that for HD.4.3 Minimum spKt/V levels for different dialysis schedules:
- 4.3.1 Two to 6 treatments per week are appropriate for certain patients.
- 4.3.2 Twice-weekly HD is not appropriate for patients with Kr less than 2 mL/min/1.73 m2.
- 4.3.3 Minimum spKt/V targets for 2-, 4-, and 6-times-per-week dialysis schedules logically should be different from that for the thrice-weekly schedule. In the absence of dose-ranging outcomes data, minimum spKt/V targets for different schedules can be based on achieving a minimum stdKt/V of 2.0 per week.
- 4.3.4 The target spKt/V dose should be at least 15% higher than the listed minimum dose because of the variability in measuring Kt/V, as discussed in Guideline 4.
4.4 RKF (measured by Kr):
- 4.4.1 The minimally adequate dose of dialysis can be reduced in patients with Kr greater than 2 mL/min/1.73 m2.
- 4.4.2 In the absence of dose-ranging outcomes data, the minimum spKt/V target for patients with substantial RKF can be reduced, but the reduced target should be no lower than 60% of the minimum target for patients with no residual renal function (the reduction depends on dialysis frequency), per values provided in Table 13.
- 4.4.3 When the minimally adequate dose is reduced because of substantial RKF, Kr should be monitored at least quarterly and as soon as possible after any event that might have acutely reduced RKF.
4.5 Increase in minimally adequate dose for women and smaller patients:
An increase in the minimally adequate dose of dialysis should be considered for the following groups of patients:
- 4.5.1 Women of any body size.
- 4.5.2 Smaller patients, for example, patients with values for anthropometric or modeled V of 25 L or lower.
4.6 Dialysis adequacy for patients who are malnourished and/or losing weight:
An increase in the minimally adequate dose of dialysis and/or a change to a more frequent dialysis schedule should be considered for the following groups of patients:
- 4.6.1 Patients whose weights are 20% less or lower than their peer body weights.
- 4.6.2 Patients with recent otherwise unexplained and unplanned weight loss.
4.7 Dialysis adequacy for patients with hyperphosphatemia or chronic fluid overload and other categories of patients who might benefit from more frequent dialysis:
A change to a more frequent dialysis schedule should be considered for the following groups of patients:
- 4.7.1 Patients with hyperphosphatemia.
- 4.7.2 Patients with chronic fluid overload with or without refractory hypertension.
4.8 A change to a more frequent dialysis schedule may be beneficial to a broader group of patients in terms of improving QOL and quality of sleep, reducing sleep apnea, and improving sensitivity to erythropoietin.
4.9 Minimum dialysis treatment time for thrice-weekly schedules:
The minimum HD treatment time for thrice-weekly dialysis in patients with Kr less than 2 mL/min should be at least 3 hours.
High-Flux Membrane (CPR 4.1)
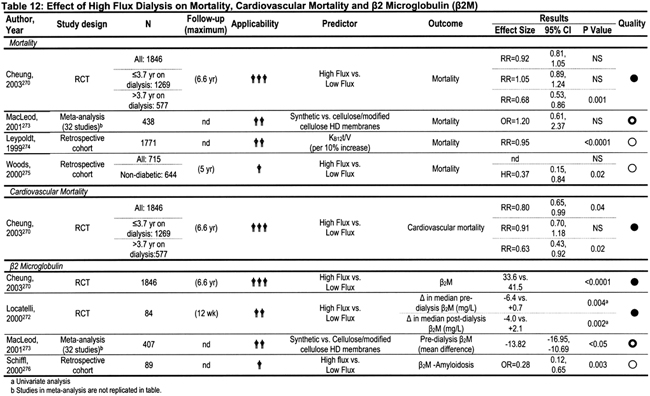
The β2M molecule has an important role in the pathogenesis of dialysis-related amyloidosis, which is seen primarily in HD patients who have been dialysis dependent for more than 5 years. An important question is whether use of membranes that clear β2M gives rise to superior outcomes over shorter periods, especially in terms of such hard outcomes as mortality and hospitalization. The primary results of the HEMO Study suggested that assignment to dialysis using a high-flux membrane had no significant effect on patient mortality or a variety of main secondary outcomes that combined mortality with either hospitalization or decrease in serum albumin levels.1 However, in contrast to results of dose randomization (for which the mean effect size of dose on mortality or secondary outcomes was close to zero) in the flux analyses, the mean effect size for mortality, as well as for several of the secondary outcomes, was fairly consistently close to a 10% benefit, although the 95% confidence intervals (CIs) included zero. Further analysis of the HEMO Study data showed that assignment to high-flux dialysis improved mortality (as well as main secondary outcomes) in higher vintage patients, ie, those dialyzed longer than the median time of 3.7 years at baseline.270 This analysis in higher vintage patients was predefined at the outset of the HEMO Study before beginning the trial. Furthermore, some of the secondary outcomes—in particular, composites focusing on cardiovascular death and/or cardiovascular hospitalizations—were improved in the group assigned to high-flux therapy.271
During the KDOQI HD update period, 2000 to 2005, no other randomized trials assessing hard end points (mortality and/or hospitalization) in patients undergoing high-flux versus low-flux dialysis were published. Several randomized trials looked at the effects of high-flux dialysis on predialysis β2M levels, and all found a measurable effect (reduction in level with high-flux dialysis), including the HEMO Study (see Table 12). 270,272,273
Additional observational studies suggested that the mortality rate might be decreased in patients dialyzing with high-flux membranes (see Leypoldt, 1999,274 and Woods, 2000275 in Table 12). After results of the HEMO Study were disclosed, analysis of mortality versus flux data from the 1999 to 2000 USRDS, published in abstract form, found a small mortality risk reduction (relative risk [RR], 0.972; 95% CI, 0.950 to 0.995) in prevalent patients, and an RR of 0.951 (CI, 0.937 to 0.966) in incident patients dialyzed with high-flux membranes.277A However, this abstract has not been published as an article in a peer-reviewed journal.
In a large European cohort of patients making up the Lombardi registry, mortality and risk for carpal tunnel surgery were compared in patients undergoing (mostly low-flux) HD, hemodiafiltration, and hemofiltration.278 A 10% mortality risk reduction was found in patients treated with either hemodiafiltration or hemofiltration compared with mostly low-flux HD, but the CI included zero. However, the investigators found a significant risk reduction for carpal tunnel surgery in the hemodiafiltration/hemofiltration groups.
The most recent European Best Practice Guidelines include recommendations for the use of high-flux membranes, supported by level B evidence (Guideline II.2.1) and also recommend the addition of a convective component to enhance middle-molecule removal, also with level B evidence (Guideline II.2.2).279 However, a recent Cochrane group review, looking at a meta-analysis of RCTs studying the effect of dialysis membrane on outcome, concluded that it was too soon to make a definitive recommendation.273
The Work Group ultimately decided that the evidence for benefits of high-flux membrane use in terms of hard outcomes was suggestive, but not definitive enough to be formulated as a guideline, taking a more conservative approach than the European group. However, the Work Group decided that the evidence for mortality reduction was strong enough for a CPR encouraging high-flux dialysis. The evidence is incontrovertible that high-flux dialysis decreases predialysis serum β2M levels (Table 12),270,272,273 and lower predialysis β2M levels were linked to improved outcome. Furthermore, reduced long-term consequences of β2-amyloidosis with the use of high-flux membranes was reported by 2 groups,280,281 confirming a much earlier report.282
The Work Group also specified a definition of high-flux dialysis. In the HEMO Study, β2M clearances were measured in vivo, and a clearance of at least 20 mL/min was defined as adequate for a dialyzer to be considered high flux (the low-flux dialyzers used had β2M clearance indistinguishable from zero). Because the manufacturing industry has learned how to expand β2M clearances while minimizing albumin leakage, current dialyzers are available with much greater β2M clearances, and the clearance can be increased still further by the use of hemodiafiltration and/or novel dialyzer designs. The value of 20 mL/min was adopted for these guidelines because it corresponded to the minimum level obtained in the HEMO Study, which provided much of the evidence for this CPR.
Minimum Dose With Hemofiltration or Hemodiafiltration (CPR 4.2)
Urea is a surrogate adequacy molecule for measuring clearance of a large family of uremic toxins, some of which may have a much higher molecular weight. Because convective removal accelerates removal of larger (>5 kd), yet permeable, solutes during extracorporeal therapy, it might be argued that with hemofiltration, the ratio of removal of these larger molecular-weight toxins to urea removal is higher; hence, minimal adequacy parameters based on urea removal either do not apply or existing minimal adequacy guidelines based on urea removal should be lower when hemofiltration is used. No dose-finding studies of hemofiltration that report hard outcomes could be identified by the Work Group. In the absence of data to the contrary, the Work Group decided to maintain recommended minimum adequacy standards for urea removal for both hemofiltration- and hemodiafiltration-based therapies. With hemodiafiltration, urea removal usually is unchanged or slightly enhanced by the supplemental filtration, so this was a somewhat moot issue. However, for some forms of primarily hemofiltration-based dialysis therapy (in which limited amounts of replacement fluid are used), the recommended minimum levels of urea removal may be difficult to achieve. The Work Group decided, on the basis of current evidence and lack of an interaction between urea-based adequacy and flux in the HEMO Study, that it would be prudent to recommend the same minimum levels of spKt/V for HD, hemofiltration, and hemodiafiltration.
Minimum spKt/V Levels for Different Dialysis Schedules (CPR 4.3)
The KDOQI 2000 HD Adequacy Guidelines gave adequacy recommendations only for thrice-weekly HD schedules. Since the last update, 1 important cross-sectional study appeared suggesting that survival in patients treated with twice-weekly HD was no worse (and was possibly better) in a USRDS patient sample.234 Given these data and with earlier initiation of dialysis in patients with higher levels of RKF, the Work Group decided that thrice-weekly HD as a minimum frequency level was no longer appropriate. Based on solute kinetics (discussed later), the Work Group was comfortable recommending a twice-weekly dialysis schedule, but only for patients with substantial RKF.
Also, since the KDOQI 2000 update, a large set of studies was published regarding the potential advantages of giving dialysis treatments more often than 3 times per week. The number of treatments ranges from an additional fourth treatment per week in patients who have problems controlling volume283 to offering short “daily” dialysis treatments ranging from 1.5 to 3 hours (or longer) 4 to 6 times per week. An alternative method of extending therapy is to greatly increase dialysis treatment time (from the usual 2.5 to 5 hours) to 7 to 10 hours by giving dialysis at night. Various frequency schedules for nocturnal dialysis have been reported, from 3 to 6 times per week.284 Simple avoidance of the 2-day interdialysis interval by giving dialysis every other day also has been advocated.285
At the time of the present guideline update, no RCTs have been conducted to measure hard outcomes (mortality and/or hospitalization) comparing conventional thrice-weekly dialysis with either short-daily or nocturnal HD. Also, no dose-finding RCTs have appeared comparing frequent short dialysis with longer nocturnal regimens in an effort to achieve varying degrees of solute removal.
Given the lack of maturity of the research data in this field, the Work Group decided to refrain from making specific recommendations about the usefulness of these therapies in terms of a guideline or from proposing guidelines regarding minimally adequate therapy given more frequently than 3 times per week.
How to measure adequacy of more frequent therapies is not established. One of the main benefits of more frequent therapies may be ridding the body of solutes that are difficult to remove, such as phosphate, β2M, or some still unknown uremic toxins. Another benefit may be in better control of salt and water balance, which may impact on patient survival as much as solute control. In particular, the Work Group was impressed with observational data linking hard outcomes to calcium-phosphorus product,286 as well as better control of serum phosphorus levels with more intensive daily dialysis schedules200 and most nocturnal dialysis schedules.284 Because 2, 4, 5, and 6 treatments per week (nocturnal and/or short-daily therapies) increasingly are prescribed, the Work Group decided that some guidance was needed in terms of minimally adequate doses.
Although an argument could be made that urea is not the only solute to use for measuring doses in a more frequent dialysis setting, control of small-solute levels in patients is vital to survival, so the Work Group decided to base recommendations for this CPR on urea. Potential alternative solutes, such as β2M, are not as clearly linked to outcome. Phosphate, while clearly linked to outcome, has complex and as yet poorly defined kinetics, and serum levels are affected not only by dialysis, but also by diet and consumption of phosphorus binders. One of the major disadvantages of urea is the rapidity of its diffusion among body compartments (high intercompartmental mass transfer area coefficient). This limitation can be minimized by using the stdKt/V construct, as described in detail in CPR 2 and in the Appendix. When the dialysis dose is expressed as stdKt/V, it seeks to control the mean pre-dialysis BUN, but, alternatively, it can be considered to model a well-cleared, but highly sequestered, solute with a low intercompartmental mass transfer area coefficient. Because highly sequestered solutes will have a large rebound after dialysis, the time-averaged blood level will be close to the mean predialysis level. stdKt/V also has the quality of reflecting advantages of a more frequent dialysis schedule that more efficiently removes sequestered solutes, such as phosphorus, but also possibly including a whole range of dialyzable solutes in the 100 to 1,000 d molecular-weight range.
In developing this CPR, the Work Group decided to target a minimum dialysis dose equivalent to an stdKt/V level of 2.0 per week. This is the level obtained when one dialyzes using a thrice-weekly schedule to an spKt/V of approximately 1.2 per treatment over 3.5 hours (Table 19).
In the absence of RKF, it is not possible to reach an stdKt/V of 2.0 by using a twice-weekly schedule. Kinetic modeling was used to examine the levels of spKt/V per treatment that would be required to reach a weekly stdKt/V value of 2.0 for twice-weekly to 7-times-weekly schedules by using dialysis treatment times ranging from 2 to 8 hours. The simulation was performed both in the absence of RKF and when Kr was 2 mL/min. This simulation was used to arrive at the recommended minimum values in Table 13.
These spKt/V values should be considered minimum values, not target values. It is especially important to note that extending dialysis time is much more effective for controlling solute levels when frequency is increased to 4 to 7 treatments per week. Particularly in short-daily therapies, longer treatment times markedly improve phosphate removal.
From Table 19, similar spKt/V values can be determined for 8-hour treatments more typical of nocturnal HD. Usually the Kt/V for an 8-hour treatment, even at reduced dialysate and blood-flow rates, will be greater than 1.0; hence, the Work Group did not believe that adequacy determined by predialysis or postdialysis BUN monitoring is appropriate for nocturnal HD schedules.
Target spKt/V Values per Treatment for More-Frequent Therapies
In contrast to thrice-weekly schedules, for which there are good data regarding the variance in Kt/V on repeated measurements, no such data have been published for short-daily dialysis, although there is no reason to assume that it would be much different from the 10% variance found in the HEMO Study. For this reason, the Work Group recommended targeting an spKt/V value that is about 15% higher than the recommended minimum targets in Table 19 in the Appendix.
Residual Kidney Function (CPR 4.4)
The KDOQI 2000 HD Adequacy Guidelines left unspecified any adequacy recommendations for patients with substantial RKF (GFR ≥ 5.0 mL/min/1.73 m2, defined as the average of urea plus creatinine clearance). Given the trends and recommendations for earlier institution of dialysis therapy and perhaps the more successful preservation of RKF in the past several years, a large number of currently dialyzed patients have substantial RKF. A consideration of solute kinetics shows that even low levels of RKF can account for removal of large amounts of solute, including such large-molecular-weight solutes as β2M, in addition to helping maintain salt and water balance. Although there are no reliable outcome data suggesting that the delivered dose of dialysis might safely be reduced in patients with substantial RKF, reduction of the extracorporeal dose makes sense from a solute-kinetics viewpoint. The HEMO Study deliberately excluded patients with Kr for urea greater than 1.5 mL/min and hence cannot be of guidance. Observational studies suggested a benefit of even small levels of RKF in terms of survival and other secondary outcome measures, so it is clear that all possible efforts should be expended to maintain RKF (see Guideline 6).
The Work Group was of the opinion that, at the present state of incomplete knowledge, the best way to adjust for residual renal urea clearance is to add it to the weekly stdKt/V. Residual urea clearance of 2 mL/min is approximately 20 L/wk of clearance; accordingly, in a patient with V = 30 L, it represents about a 0.67 weekly Kt/V unit. Table 13 shows spKt/V values per treatment corresponding to a weekly stdKt/V value of 2.0 in patients undergoing 2 to 6 treatments per week after adjusting (or not) for a weekly Kr of 2 mL/min. In discussing adjustments for Kr, the Work Group had 2 broad areas of concern.
First, the kinetic effect of RKF is so powerful that in patients with Kr greater than 2 mL/min, an equivalent reduction in spKt/V would result in very low recommended values. The Work Group believed this was undesirable for 2 reasons: (1) very low Kt/V values, especially for the twice-weekly or thrice-weekly schedules, would limit other potential beneficial effects of dialysis, including salt and water control; and (2) RKF sometimes can decrease precipitously. Patients who were receiving a markedly reduced dose of dialysis because of a higher Kr then might be underdialyzed for a few months until the reduction in Kr was recognized and acted upon. For these reasons, the Work Group developed an alternative scheme that limited the downward adjustment in spKt/V for Kr to 2 mL/min, even for patients with higher levels of Kr. The decision to “cap” the reduction in session Kt/V was based on the lack of outcomes data in patients who have higher levels of RKF and receive very low amounts of dialysis Kt/V. Maintaining a minimum “total Kt/V” value of 1.2, using an exact calculation of the required dialysis spKt/V as described in the Appendix, would allow reduction of the dialysis dose down to near zero at levels of RKF that are below the threshold for initiating dialysis. The wisdom of recommending this fully incremental approach was intensely debated in the Work Group. Opinions differed, so it was decided to leave further reductions in dialysis dose, below values suggested in Table 13, to the discretion of the clinician. One single study81, addressed this issue but there are few other studies of outcomes in patients with RKF hemodialyzed using an incremental dialysis schedule. This remains a critical area where more research is recommended.
Second, it was recommended that in patients for whom treatments are reduced because of Kr of 2.0 or greater, Kr should be rechecked at least quarterly (every 3 months) and after any event suspected to be associated with a sudden decrease in Kr. However, because the Work Group did not want to impose a burden of verifying Kr for all patients in a dialysis clinic, the recommendation is to verify it only in patients for whom the target dialysis dose is reduced.
Increase in Minimally Adequate Dose for Special Populations (CPR 4.5)
One potential area of concern relates to selected subgroups of patients who may require more dialysis. During the design phase of the HEMO Study, 7 such subgroups were postulated, including patients with high comorbidity scores, patients with diabetes, high-vintage patients, Caucasian patients, and women. Based on HEMO Study results plus results from subsequent cross-sectional studies plus clinical judgment and “common sense,” the Work Group recommended possibly increasing the target dose of dialysis in 2 groups of patients: women and small patients.
Women
Of the 7 “high-risk” groups identified during the design phase in the HEMO Study, an interaction with dose group assignment was present for only women (Table 8).13 Women assigned to the higher dose of dialysis (URR ~75%, on average) had better survival than those assigned to URR of about 63%. The overall benefit for men and women was close to zero because an opposite nonsignificant trend for increased mortality in men assigned to the higher dose of dialysis also was found. As best could be determined, the sex-dose-mortality interaction was not caused by body size, although most women in the HEMO Study had a smaller body size, determined by using a variety of measures, with little overlap with the men in the study. While the HEMO Study was in progress, others identified a similar sex-dose interaction,57 and after HEMO Study results were reported, another group reported a similar association in the USRDS-Medicare database.104
To complicate matters, the dose-targeting bias (discussed in more detail in Guideline 4) appeared to be enhanced in women compared with men.98 This means that observational data should not necessarily be considered confirmatory of the intent-to-treat sex-dose-mortality interaction identified in the HEMO Study. However, because both randomized and observational data suggested that a higher dose of dialysis might be beneficial for women, the Work Group was comfortable with issuing a CPR for considering a higher dialysis target dose in women. For the most part, this happens naturally because most women have a smaller value for V; thus, the same prescription applied to a man and a woman, even considering patients of equal weight, will result in a higher Kt/V in the woman.
Body Size
There are, of course, multiple reasons why a patient can be “small.” A patient can be short, small boned, or simply thin, all without being malnourished. Most data examining body size versus dose versus mortality interactions looked at anthropometric measures in which body size was derived from weight and height—eg, body mass index (BMI)—and, in some studies (in which Watson V was used), sex, and age. It appears that most of the mortality effect in these studies is related to BW because the Work Group was not able to find data in which patient height was a predictor of mortality (nor was height a predictor of mortality in the HEMO Study). It is then presumed that patients with lower BMI or Watson V primarily are underweight patients who are malnourished.
A separate issue is whether smaller nonundernourished patients who are at or near their expected weight might require more dialysis. Here, the argument has to do with sizing delivered dose of therapy based on body water, which is a factor of BW to the 1.0 power (usually V = some factor multiplied times the postdialysis weight). GFR usually is sized according to BSA, which is a factor multiplied times BW raised to the 0.667 (2/3) power. If Kt was normalized to BSA or some factor multiplied by V0.667 and a single target value was assigned for all values of weight, the result would be that more dialysis would be assigned to smaller patients than with the current Kt/V strategy, and less dialysis would be assigned to very large patients. The argument has been made that V is determined substantially by skeletal muscle mass, which may be relatively quiescent in terms of generation of uremic toxins. Although women or less muscular men may have a smaller V than similar-height controls, it does not necessarily mean they require less dialysis.
The Work Group noted and reviewed a number of studies in this field, examining the relationship of Kt and various measures of body size. Most of these analyzed the Fresenius North America patient data set.78,101
The Work Group also looked at an analysis of survival by various body size parameters in the HEMO Study,13 in which various measures of body size were not found to interact with delivered dose. The Work Group concluded that there was not sufficient evidence to abandon the concept of sizing of dialysis dose according to V for the moment because cross-sectional survival analyses of dose versus mortality have so many biases that—at present—the effects of individual confounding factors have not been completely clarified. Furthermore, there is great simplicity in being able to monitor delivered dialysis dose based on URR and then combine this with weight loss and other information to compute a delivered Kt/V.
The compromise solution for the present update was to keep the dose as spKt/V and the minimum dose unchanged, as per the KDOQI 2000 guidelines, but to issue this CPR, which recommends that one consider increasing minimum dialysis dose targets in both women and small patients.
Several logical questions arise:
The Work Group decided to leave these decisions up to the practitioner, although an increased minimum dose of 25% was the range of increase in dose envisaged for either women or small patients (eg, to an spKt/V of 1.5 for a thrice-weekly schedule with Kr< 2).
Dialysis Adequacy for Patients Who Are Malnourished and/or Losing Weight (CPR 4.6)
Because nutrition tends to deteriorate even at relatively well-preserved levels of renal function,288 the notion is prevalent in the dialysis community that increasing the amount of dialysis may help improve nutritional status. A variety of nutritional parameters were measured in the HEMO Study, and the higher-dose group did not show improvement in any of the nutritional parameters measured, including serum albumin, anthropometrics, or food intake. However, patients treated with longer (8-hour) periods of dialysis given 3 times per week or patients following 6-times-per-week short-daily dialysis regimens or nocturnal-dialysis regimens sometimes reported marked benefits in terms of food intake, serum albumin level (although this is confounded by blood volume changes caused by hemoconcentration), and increase in dry BW.284
For these reasons, the Work Group issued the present CPR, which recommends that practitioners consider increasing the dose of dialysis in a thrice-weekly framework in patients who are judged to be malnourished by BW criteria, subjective global assessment, or other means. The lack of a beneficial effect on nutritional parameters in the HEMO Study of increasing spKt/V from 1.3 to 1.7 suggests that perhaps a more useful strategy in such patients is to increase dialysis frequency, although it is recognized that such therapies are not uniformly available at all centers.
Dialysis Adequacy for Patients Who Are Hyperphosphatemic or With Refractory Volume Overload and Other Categories of Patients Who Might Benefit From More Frequent Dialysis (CPR 4.7)
Patients With Hyperphosphatemia
Serum phosphorus level appears to be a robust predictor of mortality in dialysis patients, as well as patients with CKD.286 Phosphorus control is dependent on phosphorus intake, compliance with phosphorus-binder intake, and HD prescription. Because serum phosphorus level decreases to a low level early in dialysis, increases in Kt/V in a thrice-weekly framework while holding treatment time constant (eg, by increasing blood flow rate or dialyzer urea clearance) or slight increases in dialysis treatment time are expected to have only a mild to negligible effect on serum phosphorus levels. With short-daily dialysis schedules, the initial 30 minutes of each treatment occurs while serum phosphorus levels are still high, but overall serum phosphorus control has been disappointing, especially using short (1.5- to 2-hour) treatments. Patients undergoing short-daily dialysis sometimes increase their food or protein (and therefore phosphorus) intake, which may compensate or even override the small additional amount of phosphorus removal. A recent nonrandomized study in which 3-hour treatments were given 6 times per week showed a decrease in serum phosphorus levels.200 However, it is not clear to what extent patients would tolerate 3-hour treatments given 6 days per week or if alternative measures to control serum phosphorus might be equally or more effective.
An increase in total weekly hours of dialysis, probably more than 24 h/wk, distributed over at least 3 treatments per week appears to be needed to control phosphorus levels in most dialysis patients. In the Tassin experience (8 h/wk × 3 = 24 h), approximately one third of patients no longer required phosphate binders (B. Charra, personal communication, February 2005). Using an “every-other-night” nocturnal dialysis strategy (~28 h/wk) should give results similar to those in the Tassin experience. Nocturnal dialysis given 5 to 6 times per week appears to remove the need for phosphorus binders, adequately controls phosphorus levels in almost all patients, and often requires the addition of phosphorus to the dialysate to prevent hypophosphatemia.284
Volume-Overloaded Patients
Control of patient volume and blood pressure are reviewed in detail in Guideline 5. In addition to the recommendations discussed in Guideline 5 regarding sodium balance, one of the most reliable methods to help achieve volume control is to extend total weekly dialysis time. In cases in which this cannot be done practically in a thrice-weekly framework, a 4-times-per-week schedule has proved useful. Additional benefits may be obtained by moving to a short-daily or not-so-short daily 6-times-per-week schedule, and ultimate control would be expected using a nocturnal HD schedule.
Other Categories of Patients for Whom More Frequent Dialysis May Be Beneficial
At the present time, other patient subgroups that might benefit from more frequent dialysis are not as clearly identified. It remains possible that almost all patients might benefit, although practical and reimbursement issues, as well as the present incomplete state of knowledge, clearly preclude such a recommendation. Small largely uncontrolled studies suggest that—in addition to improved nutritional status, serum phosphorus, and volume control—more frequent dialysis may improve erythropoietin sensitivity, quality of sleep, and sleep apnea, as well as overall QOL.
The Minimum Dialysis Treatment Time for 3 Treatments per Week With Kr Less Than 2 mL/min Should be 3 Hours (CPR 4.8)
This guideline evolved from 2 considerations. The first is the concept of attempting to maintain stdKt/V close to 2.0 per week as a minimum amount of dialysis across all schedules. For a 2-hour dialysis treatment, an spKt/V of at least 1.4 is required to achieve an stdKt/V of 2.0. The second consideration is that it is difficult to achieve good control of salt and water balance with very short treatment times. The outcomes evidence for this CPR is not particularly strong; in the HEMO Study, the minimum treatment time was 2.5 hours and there was no randomized evaluation of treatment time; thus, the HEMO Study is not applicable here. A study that compared conventional dialysis (3- to 4-hour treatments) with ultrashort high-efficiency hemodiafiltration found no difference in level of blood pressure control.289
Very recent studies, including 1 RCT, suggested that dialysis treatment time has an impact on outcomes.72A Cross-sectional data showed that dialysis treatment time was related inversely to mortality, but much of this effect disappeared when patient BSA was included in the model.101 It was the Work Group's belief that a minimum treatment time of 3 hours reflects clinical practice and was especially important in patients with a low Kr (<2 mL/min).
Given the difficulty conducting RCTs in the HD population, many of the questions addressed by the present CPRs will not be answered definitively with Level A evidence for many years. It takes approximately 2,000 patients to run a randomized trial powered to detect a change in mortality (eg, the HEMO trial), and even then, the power to detect smaller effects is limited.
The level of β2M clearance in the HEMO Study was modest, and it is unclear whether more definitive benefits of convective and/or high-flux treatment might be seen with high-substitution volume hemodiafiltration, in which levels of β2M clearance substantially greater than those obtained in the HEMO Study can be achieved.
The Work Group believes that given the dose-targeting bias identified in the HEMO database98 and the multiple confounding factors present in assignment of dialysis dose, modeled volume, and different survival effects caused by body size, it is difficult to draw valid conclusions about how best to target dialysis therapy based on body size. The present guidelines address the issue of increasing the amount of minimal dialysis for smaller patients. They do not address the issue of reducing the amount of minimal dialysis for very large patients, for which technical and time issues become burdensome for both staff and patient.
With regard to more frequent therapies, the Work Group understands that their use is growing markedly. The present time should be one of experimentation in terms of finding the best combination of schedules and treatment times, and the Work Group was accordingly restrained in terms of its recommendations for how best to deliver such therapies.