Identifying anemia is the first step in evaluating the prognostic, diagnostic, and therapeutic significance of anemia in the patient with CKD.
1.1.1 Stage and cause of CKD:
In the opinion of the Work Group, Hb testing should be carried out in all patients with CKD, regardless of stage or cause.1.1.2 Frequency of testing for anemia:
In the opinion of the Work Group, Hb levels should be measured at least annually.1.1.3 Diagnosis of anemia:
In the opinion of the Work Group, diagnosis of anemia should be made and further evaluation should be undertaken at the following Hb concentrations:
• <13.5 g/dL in adult males.
• <12.0 g/dL in adult females.
Anemia develops early in the course of CKD and is nearly universal in patients with CKD stage 5.17 The purpose of specifying Hb level thresholds to define anemia is to identify patients who are most likely to show pathological processes contributing to a low Hb level and who therefore are most likely to benefit from further anemia evaluation. Thus, the current guideline (Guideline 1.1) identifies Hb level thresholds that should trigger diagnostic evaluation (Guideline 1.2). Conversely, Hb levels that should trigger ESA therapy are defined in Guideline 2.1.
Stage and Cause of CKD
Hb testing should be carried out in all patients with CKD, regardless of stage or cause. Anemia is associated with CKD of any cause,18-22 including transplant-associated CKD (see Section IV). The severity of anemia in patients with CKD is related to both the degree of loss of glomerular filtration rate (GFR) and the cause of kidney disease. The lowest Hb levels are found in anephric patients and those who commence dialysis therapy at very low levels of kidney function.23,24
The reported prevalence of anemia by CKD stage depends in large part on the size of the study; whether study participants are selected from the general population, are at high risk for CKD, or are patients already under a physician’s care; what level of Hb is defined as constituting anemia; and whether patients do or do not have diabetes.
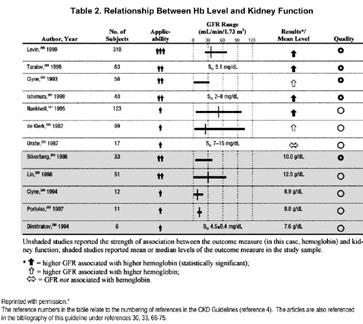
Studies reviewed for the purposes of guideline statement 1.1.1 include those of patients with CKD before dialysis therapy, those with kidney transplants, and those on dialysis therapy. The reviewed literature spans close to 40 years of investigation up to the year 2000 and describes clinical findings of researchers as they explore the relationships between Hb level or hematocrit (Hct) and kidney function (Table 2 and 3). The majority of available data were derived from studies of small sample size, most of which are cross-sectional studies, or baseline data from clinical trials of variable size and robustness.
In 12 of the 21 studies reviewed, there was an association between level of Hb or Hct and the selected measure of kidney function. They also demonstrate variability in levels of Hb or Hct at each level of kidney function, whether assessed by using serum creatinine (SCr) concentration, creatinine clearance (CCr), or estimated GFR (eGFR). However, the consistency of the information they provide indicates a trend toward lower Hb levels at lower levels of GFR and variability in Hb levels across GFR levels.
More recent studies used large databases to examine the relationship between Hb level or Hct and kidney function (Table 4). A cross-sectional analysis examined 12,055 ambulatory adult patients (≥18 years of age) who had at least 2 SCr measurements 2 years apart and at least 1 Hct measurement and weight recorded between 1990 and 1998.25 Results should progressively lower Hct at an estimated CCr less than 60 mL/min/1.73 m2 in men and 40 mL/min/1.73 m2 in women (Table 5). When GFR normalized to body surface area (BSA) was estimated by using the Modification of Diet in Renal Disease (MDRD) equation, both men and women developed a statistically significant decrease in Hct at the same BSA-normalized GFR, 50 mL/min/1.73 m2. This relationship persisted when analyses were restricted to patients who were normochromic and normocytic, ie, presumably non–iron deficient. However, the change in age- and race-adjusted Hct with decreasing levels of GFR was greater in men than women. It should be noted that only 10% of men and 6% of women had an eGFR of 50 mL/min/1.73 m2 or less (MDRD formula), and the indications for measuring SCr and Hct were unknown.
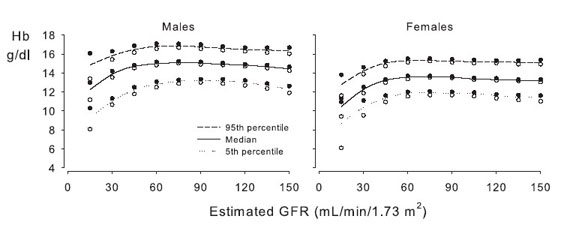
Two studies examined the relationship between prevalent Hb concentration and GFR by using results from the National Health and Nutrition and Examination Survey (NHANES) III database. NHANES III is a cross-sectional survey of nutritional and health status in 15,419 individuals randomly selected from the general US population from 1988 to 1994. Results from NHANES III26,27 are consistent with those obtained in ambulatory adult patients (Table 6). 25 At an eGFR less than 75 mL/min/1.73 m2 in men and 45 mL/min/1.73 m2 in women, the associated Hb level is lower than that seen at eGFRs above those thresholds (Fig 3). The relationship is observed best in the distribution of the lowest fifth percentile of Hb levels. In general, the lower the eGFR at less than the respective threshold, the lower the associated Hb level, but confidence intervals (CIs) are broad at the lowest eGFR levels because the number of observed individuals with a low eGFR is small. For example, only 3.5% of women and 1.4%; of men in the survey showed an eGFR of 30 mL/min/1.73 m2 or less.
The relationship between GFR and prevalence of anemia is determined in large part by the Hb concentration used to define anemia. In NHANES III, prevalence of a Hb level less than 13 g/dL increases at less than a threshold eGFR of 60 mL/min/1.73 m2 in males and 45 mL/min/1.73 m2 in females (Fig 4). However, the prevalence of a Hb level less than 11 g/dL is not greater than reference except at an eGFR less than 30 mL/min/1.73 m2 in both males and females. Again, precise estimates of prevalence are limited by relatively small numbers; in particular, less than a GFR of 30 mL/min/1.73 m2 (N = 52).
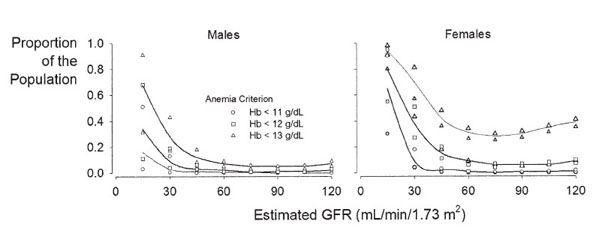
Fig 4. Relationship between eGFR and prevalence of anemia as defined by differing Hb levels for (A) males and (B) females. Data from NHANES III. Reprinted with permission.27
The relationship between prevalence of anemia and GFR may be changing as a result of earlier identification and treatment of anemia. Comparing results of NHANES III with NHANES IV shows a lower prevalence of anemia for each CKD stage in the more recent survey (Fig 5). 28 In this analysis, anemia was defined by World Health Organization (WHO) criteria (Hb level < 12 g/dL in women and < 13 g/dL in men).
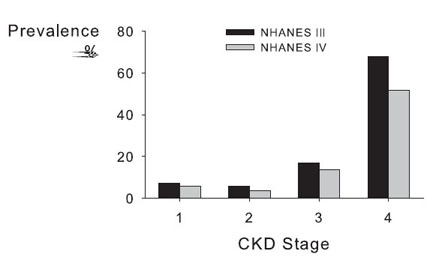
Fig 5. Prevalence of anemia by CKD stage. Anemia, defined as an Hb level less than 13.0 g/dL in males and less than 12.0 g/dL in females, was plotted by stage of CKD in results from NHANES III compared with NHANES IV. USRDS 2004.28
The prevalence of anemia among patients is much greater than that observed among individuals randomly surveyed in the general population. In a cross-sectional study of 5,222 adult patients with CKD who were selected from 237 US physician practices (including family practice, internal medicine, nephrology, and endocrinology), mean Hb levels for an MDRD eGFR of 60 or greater (CKD stages 1 and 2), 30 to 59 (CKD stage 3), 15 to 29 (CKD stage 4), and less than 15 mL/min/1.73 m2 (CKD stage 5) were 12.8 ± 1.5, 12.4 ± 1.6, 12.0 ± 1.6, and 10.9 ± 1.6 g/dL, respectively.29 The prevalence of untreated anemia (defined as a Hb level < 12, 10 to 12, and < 10 g/dL) for different CKD stages (Fig 6) is much greater than that reported in NHANES surveys.26,27 A greater disease burden among patients compared with randomly selected individuals provides a likely explanation for these findings.
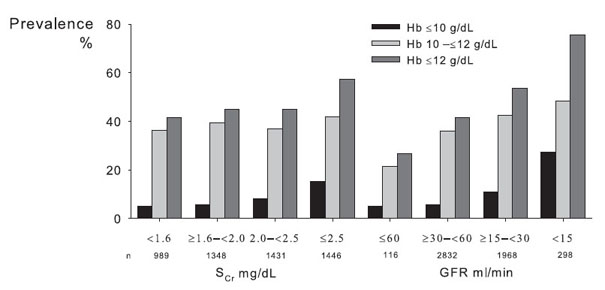
Fig 6. Relationship between level of renal function, reflected by SCr level or GFR, and prevalence of anemia, defined at different Hb cutoff levels, among patients under the care of physicians. Reprinted with permission.29
Because data points at GFR levels less than 30 mL/min/1.73 m2 were scarce in all except the previous cross-sectional study,29 the Canadian Multicentre Study30 was used to demonstrate trends in a large cohort of patients before dialysis therapy (Fig 7). The prevalence of anemia was greatest at the lowest levels of GFR, but approached 20% among patients with a GFR of 30 to 44 mL/min/1.73 m2 (Fig 8). Similarly, in another study of 131,848 patients who began dialysis therapy in the United States between 1995 and 1997, proportions of patients with Hcts less than 30% and less than 36% immediately before the initiation of dialysis therapy were 67.5% and 93%, respectively.31
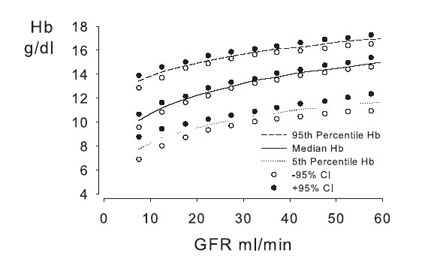
Fig 7. Hb percentiles by GFR in the Canadian Multicentre Longitudinal Cohort Study. Patients were referred to nephrologists between 1994 and 1997. Results shown exclude patients receiving ESA therapy or with an arteriovenous fistula. Reprinted with permission.4 ,30
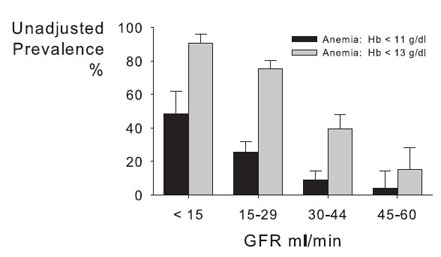
Fig 8. Prevalence of low Hb level by category of GFR in the Canadian Multicentre Longitudinal Cohort Study. Reprinted with permission.4 ,30
Patients with diabetes are more prone to both develop anemia and develop anemia at earlier stages of CKD than their nondiabetic counterparts (Fig 9). 32-37 In a cross-sectional clinical audit of 820 Australian patients with diabetes, anemia was 2 to 3 times more prevalent in patients with diabetes compared with the general population at all levels of GFR (Fig 9). In an extension of the same audit, patients with type 2 diabetes with a CCr of 60 to 90 mL/min/1.73 m2 were twice as likely to have anemia than those with a CCr greater than 90 mL/min/1.73 m2. Patients with diabetes with a CCr less than 60 mL/min/1.73 m2 were twice as likely to have anemia as those with a CCr of 60 to 90 mL/min/1.73 m2.36 In the Kidney Early Evaluation Program (KEEP 2.0), a cross-sectional community-based screening program aimed at detecting CKD among high-risk patients (those with diabetes, hypertension, or a family history of kidney disease), the prevalence of anemia at each level of eGFR from patients with CKD stages 2 through 5 was greater than in patients without diabetes: 8.7% versus 6.9% in stage 2 (P = not significant [NS]), 7.5% versus 5.0% in stage 3 (P = 0.015), 22.2% versus 7.9% in stage 4 (P < 0.001), and 52.4% versus 50% in stage 5 (P = 0.88).37
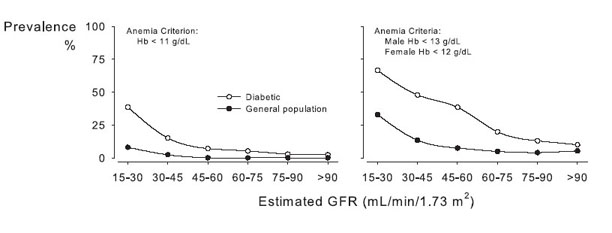
Fig 9. Prevalence of low Hb level by eGFR in patients with diabetes compared with the general population. Open circles indicate the diabetic population, while closed circles represent the general population data from NHANES III. A: Hb ≤11 g/dL; B: WHO criteria (men, Hb <13 g/dL; women, Hb <12g/dL). Reprinted with permission.35
The following conclusions can be drawn from these studies: (1) the prevalence of anemia at higher levels of GFR (CKD stages 1 and 2) is relatively low in individuals randomly selected from the general population, but is not uncommon in patients with CKD under the care of a physician or identified by virtue of being at high risk for CKD; (2) among individuals from a general population, mean Hb levels decrease and anemia develops consistently only when GFR is less than 60 mL/min/1.73 m2 (stage 3 CKD); (3) the prevalence of anemia increases at later stages of CKD (CKD stages 4 and 5); (4) there is significant variability in Hb levels at any given level of kidney function; and (5) anemia among patients with diabetes compared with patients without diabetes is more prevalent, more severe, and occurs earlier in the course of CKD.
These observations underscore the need to measure Hb levels in every patient with CKD, regardless of stage or cause.
Frequency of Hb Testing in Patients With CKD
Hb levels should be measured at least annually. Because little is known about the natural history of anemia in patients with CKD, precise information is unavailable to determine the optimum frequency of Hb testing in patients with CKD. The recommendation that patients be evaluated at least annually rests on observations from clinical trials that (in the absence of ESA therapy) the natural history of anemia in patients with CKD is a gradual decline in Hb levels over time.38,39 In 1 trial,39 patients assigned to the lower Hb target (9.5 to 10.5 g/dL) entered the trial with Hb values greater than target (mean, 11.8 g/dL) and received ESA only if Hb levels subsequently decreased. During 2 years, a significant proportion of patients eventually required ESA therapy. However, among those who did not require ESA therapy, mean Hb values remained relatively stable (Fig 10). This evidence suggests the need for regular surveillance of Hb levels in patients with CKD without ESA therapy. The statement Hb levels should be measured at least annually emphasizes that more frequent Hb surveillance likely will be needed for selected patients, including those with greater disease burden, unstable clinical course, or evidence of previous Hb level decline. Frequency of Hb testing in patients already undergoing ESA therapy is addressed in Guideline 3.1.1.
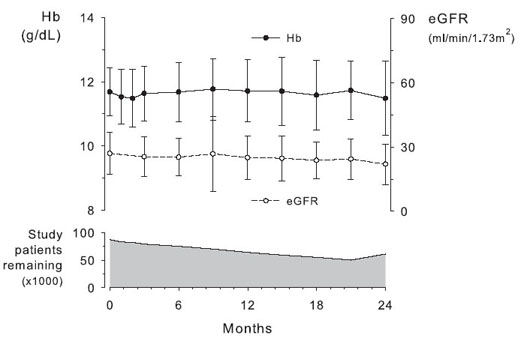
Fig 10. Changes in Hb levels and eGFR over 2 years in adult patients with CKD stages 3 and 4 not treated with ESA. Oral iron supplements and occasional IV iron treatment were administered to maintain TSAT at greater than 20% and ferritin levels greater than 100 ng/mL. Data given as mean ± SD. Although mean Hb levels showed little or no change, the number of study patients remaining without ESA therapy declined. Results courtesy of the authors, from control group patients previously described. Reprinted with permission.39
Diagnosis of Anemia
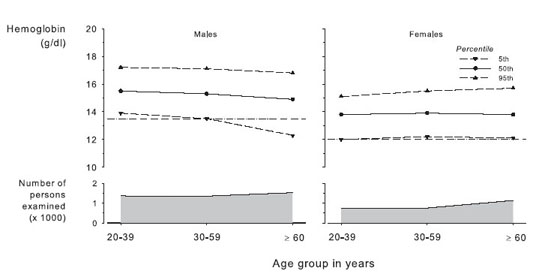
Diagnosis of anemia should be made and further evaluation should be undertaken at Hb concentrations less than 13.5 g/dL in adult males and less than 12.0 g/dL in adult females. The recommended thresholds for defining anemia represent the mean Hb of the lowest fifth percentile of the sex-specific general adult population (Table 7 and 8; Fig 11). 40,41 The conclusion that anemia therefore is defined as a Hb level less than 13.5 g/dL in adult males and less than 12 g/dL in adult females (Fig 3) assumes a lack of adjustment downward for age in males and an adjustment upward for iron deficiency in females. Although mean Hb level representing the fifth percentile decreases among males older than 60 years, it is clear that a substantial fraction of older males with low Hb levels show concurrent evidence of pathological conditions that may cause or contribute to anemia.32 Because we cannot exclude potential pathological states, we cannot assume that lower Hb levels in older males are normal. Therefore, we make no adjustment for age among males. The recommended threshold of less than 12.0 g/dL in females reflects exclusion from the healthy population of individuals whose iron status test results suggest that iron deficiency is contributing to a low Hb level.
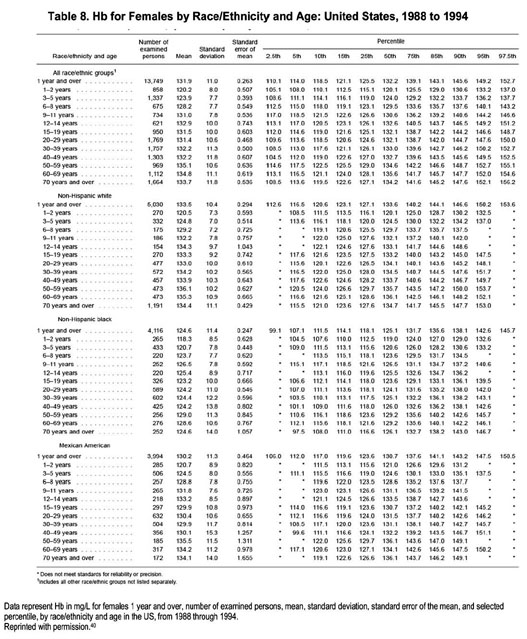
The recommended definition of anemia differs from previous CPGs. The WHO defines anemia as a Hb level less than 13.0 g/dL in adult men and less than 12 g/dL in adult women.42 Differences between the current recommendation and the WHO definition arise from differences in the data source for the general population: the WHO definition is based on sparse data obtained prior to 1968, whereas the definition proposed in the current guidelines is based on more recent NHANES III data. The recently published Revised EBPG for the Management of Anaemia in Patients with Chronic Renal Failure also defines anemia as a Hb level less than 13 g/dL in men, but adjust this to less than 12 g/dL for men older than 70 years. Because the EBPGs do not adjust for the potential contribution of iron deficiency in women, the EBPG threshold for women is less than 11.5 g/dL.16 Finally, the previous KDOQI Anemia Guidelines recommended less than 12 g/dL for men (reflecting use of a different data set), less than 11 g/dL for women of reproductive age (different data set, no attempt to exclude effect of iron deficiency), and less than 12 g/dL for postmenopausal women (no change).
The importance of identifying patients with anemia in the presence of CKD is 2-fold. First, a Hb value in the lowest fifth percentile of the general population may signify the presence of significant nutritional deficits, systemic illness, or other disorders that warrant attention. Second, anemia in patients with CKD is a known risk factor for a number of significant adverse patient outcomes, including hospitalizations, CVD, cognitive impairment, and mortality.16,43-50 Regardless of whether concurrent disorders, if any, are treatable, awareness of their presence is likely to be helpful to the clinician. Similarly, the benefits of anemia treatment (see Guideline 2.1) cannot extend to patients whose anemia remains unidentified.
Altitude, age, race, and smoking each contribute to the interpretation of the normal range of Hb values, in addition to sex, and therefore must be considered in patients with CKD. The current definition for anemia reflects results from adult patients older than 18 years, of all races and ethnic groups, and living at relatively low altitude (<1,000 m or 3,000 ft).
Altitude has a direct impact on red blood cell number, mass, and volume.51 Generally, Hb concentration can be expected to increase by about 0.6 g/dL in women and 0.9 g/dL in men for each 1,000 m of altitude above sea level.52 This increase in Hb levels seems to be caused at least in part by increased erythropoietin production.53 The threshold Hb level defining anemia in patients living at high altitude should be adjusted upward, in keeping with the degree of elevation (Table 9).
In children, mean Hb level and the variability around the mean vary highly with age. These findings are discussed elsewhere in more detail (in CPR for Children 1.1). Among adult males, as previously noted, the lower fifth percentile of Hb values declines as age advances (Fig 11). One study reported a mean decrease of 1 to 1.5 g/dL in Hb concentration in males as they increased in age from 50 to 75 years; however, this trend was not seen in women, in whom Hb concentration remained stable between 20 and 80 years of age.54 Although it previously was believed that decreases in Hb levels might be a consequence of normal aging, evidence has accumulated that anemia reflects poor health and increased vulnerability to adverse outcomes in older persons.32 Consequently, the current recommendation makes no adjustment for aging in men. An association between an increasing prevalence of anemia and older age has been reported in most,27,55 but not all,29 studies of patients with CKD, in keeping with current opinion that anemia is not a normal consequence of aging.56
Among women of reproductive age, menstrual losses, the potential for iron deficiency, and the effect of pregnancy each deserve consideration. Although Hb values in women are lower than those in men (Table 7 and 8), Hb concentrations in iron-replete women remain stable between 20 and 80 years of age.54 Regardless of the presence or absence of CKD, the prevalence of anemia is greater in women compared with men.55 Menses and pregnancy may each contribute to this finding. Menstrual losses of iron average 0.3 to 0.5 mg/d and may result in mostly iron-deficiency anemia.42 Among pregnant women, Hb concentration decreases during the first and second trimesters largely because of the dilutional effect of expanding blood volume. In the third trimester, Hb concentration remains low in pregnant women who do not take iron supplements, but gradually increases toward prepregnancy levels in those who take iron supplements.57-59 Table 10 shows maximum Hb cutoff values for anemia during pregnancy.
Hb values also vary significantly between races, with African-American individuals consistently showing Hb concentrations 0.5- to 0.9-g/dL lower than those of Caucasoid or Oriental populations.60-62 Because the reason for this disparity in Hb level distributions by race has not been determined and could reflect increased disease burden, this guideline does not provide race-specific cutoff values for anemia.41,63,64 Anemia may develop at an earlier stage in the course of CKD among non-Hispanic African Americans compared with individuals of other races.27 African-American patients have a greater prevalence of anemia at every stage of CKD compared with whites.55
Last, smoking is associated with elevated carboxyhemoglobin levels, which are associated with a compensatory increase in total Hb concentration.65 If the same definition of anemia is applied to both smokers and nonsmokers, the risk for developing anemia is lower in current or past smokers than nonsmokers.55 Because approximately 20% to 40% of patients with CKD are current or past smokers,55 consideration should be given to the significance of a smoking history in interpreting results of Hb measurement (Table 11).
Many studies that examined the relationship between Hb level and kidney function: