NKF KDOQI GUIDELINES
KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease
EXECUTIVE SUMMARY
INTRODUCTION
Anemia commonly contributes to poor quality of life (QOL) in patients with chronic kidney disease (CKD). Fortunately, among the disorders that may afflict patients with CKD, anemia is perhaps the most responsive to treatment. Anemia was the subject of one of the first efforts of the National Kidney Foundation (NKF) to improve patient outcomes through the development, dissemination, and implementation of Dialysis Outcomes Quality Initiative (DOQI) Clinical Practice Guidelines.1 The first update of these guidelines appeared in 2001 under the newly organized NKF-Kidney Disease Outcomes Quality Initiative (KDOQI).2 In 2004, the NKF-KDOQI Steering Committee appointed a Work Group and Evidence Review Team (ERT) to undertake the first comprehensive revision of the KDOQI Clinical Practice Guidelines for the Management of Anemia in CKD. This Executive Summary provides a brief background description of CKD and anemia, outlines the scope of the guidelines and the methods of evidence review and synthesis, and provides the complete text of the guideline statements.
BACKGROUND
About CKD
CKD is a worldwide public health issue.3 In the United States, the incidence and prevalence of kidney failure are increasing (Fig 1), outcomes are poor, and the cost is high.4 The prevalence of earlier stages of CKD is approximately 100 times greater than the prevalence of kidney failure, affecting almost 11% of adults in the United States.4,5 There is growing evidence that some of the adverse outcomes of CKD can be prevented or delayed by preventive measures, early detection, and treatment. Strategies to improve outcomes include Clinical Practice Guidelines (CPGs) for CKD4 and for the management of hypertension,3 dyslipidemia,6 bone disease,7 nutrition,8 and cardiovascular disease (CVD)9 in patients with CKD.
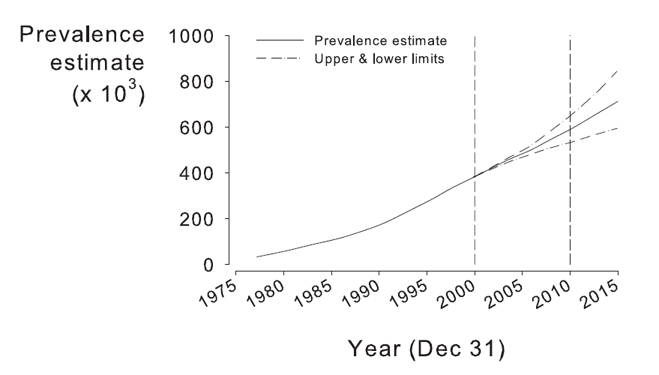
Fig 1. Kidney failure in the United States. Prevalence estimates of kidney failure treated by dialysis and transplantation (end-stage renal disease [ESRD]) in the United States. Prevalence refers to the number of patients alive on December 31st of the year. Upper and lower estimates reflect the effect of potential changes in ESRD incidence, diabetes prevalence, ESRD death rate, and underlying age and race structure of the US population after the year 2000. Reprinted with permission.10
About Anemia
Before considering a patient with both anemia and CKD, a brief introduction to the processes that contribute to anemia may be helpful. Anemia is the clinical manifestation of a decrease in circulating red blood cell mass and usually is detected by low blood hemoglobin (Hb) concentration. The cause, treatment, and prognostic significance of anemic disorders vary widely. Causes are distinguished clinically by markers of the magnitude and appropriateness of a marrow response to anemia. Under usual conditions, bone marrow generates approximately 200 billion new cells per day to match the number of senescent cells removed from circulation. The expected compensatory response to anemia is a heightened rate of erythropoiesis. Failure to demonstrate a compensatory response signifies slowed or defective erythropoiesis. Thus, hyperproliferative disorders reflect increased destruction of red blood cells with normal marrow response, whereas hypoproliferative and maturation disorders reflect impaired red blood cell production.
The cellular and molecular biology of erythropoiesis has important implications for understanding, evaluating, and treating anemia in patients with CKD. Effective circulating red blood cell mass is controlled by specialized interstitial cells in the kidney cortex that are exquisitely sensitive to small changes in tissue oxygenation.11 If tissue oxygenation decreases because of anemia or other causes, these cells sense hypoxia and produce erythropoietin.12 Within erythroid islands, the autonomous unit of erythropoiesis in marrow, receptors on the surface of the earliest red blood cell progenitors, erythroid colony-forming units (CFU-Es), bind erythropoietin. Binding of erythropoietin to erythropoietin receptors salvages CFU-Es and the subsequent earliest erythroblast generations from preprogrammed cell death (apoptosis), thereby permitting cell survival and division and the eventual expansion of erythropoiesis. If successful, these erythropoietin-stimulated events increase the production of reticulocytes, restore normal circulating red blood cell mass, and correct tissue hypoxia.13
In anemic patients, 1 or more steps in this autoregulatory sequence may fail. In the presence of kidney disease, erythropoietin production may be impaired, leading to erythropoietin deficiency and the apoptotic collapse of early erythropoiesis. If folate and vitamin B12 are lacking, deoxyribonucleic acid (DNA) synthesis is impaired, and erythroblasts, normally undergoing rapid division during this period of erythropoiesis, succumb to apoptosis.13 In ongoing folate or vitamin B12 deficiency, disordered DNA synthesis, maturation arrest, and ineffective early erythropoiesis lead to a macrocytic anemia. Conversely, if iron is lacking, the Hb-building steps that follow rapid cell division are affected: synthesis of both heme and globin slow, and erythropoiesis is impaired. Reticulocytes that emerge from marrow are few, poorly hemoglobinized, and small. A hypochromic microcytic anemia eventually results. Inflammation, a disorder common among patients with CKD, appears to impair both the early erythropoietin-dependent period of erythropoiesis and the later iron-dependent period. Inflammatory cytokines inhibit erythropoietin production, directly impair growth of early erythroblasts, and—especially in the absence of erythropoietin—promote death by ligand-mediated destruction of immature erythroblasts.14 In addition, inflammatory cytokines stimulate hepatic release of hepcidin, which simultaneously blocks iron absorption in the gut and iron release from resident macrophages, prompting a decrease in transferrin saturation (TSAT) and thereby promoting iron-deficiency erythropoiesis.15 Thus, the anemia of inflammation characteristically is hypoproliferative and not infrequently includes features suggesting iron-deficiency erythropoiesis.
This synopsis provides an introduction to the subject and purpose of the following CPGs for patients with both anemia and CKD. Guideline 1.1 describes identification of the patient with anemia and CKD. Guideline 1.2 describes the recommended initial evaluation; Guideline 2.1, the goal of treatment; and Guidelines 3.1, 3.2, and 3.3, the use of therapeutic agents.
SCOPE OF THE GUIDELINES
New findings, new agents, and the need for an expanded scope prompt the need for a comprehensive revision of existing NKF-KDOQI CPGs for the Treatment of Anemia in CKD. In preparing the current guidelines, the Anemia Work Group members broadened our inquiry to include all stages of CKD, identify areas of concern to current practitioners, adopt a structured intensive evidence review process not previously used, apply that process to both newly available literature and literature examined in the development of previous guideline versions, formulate conclusions that distinguish evidence-based guidelines from expert-opinion–based clinical practice recommendations (CPRs), and present both guidelines and recommendations in a new format to more clearly describe what is not known. To ensure that the next update profits from evidence we currently lack, we identified limitations of currently available evidence and, in a subsequent report, will identify priorities for needed research.
Intended Reader
Our intended reader is the practitioner who manages patients with CKD, including nephrologists, primary-care providers, cardiologists, nurse practitioners, nurses, and dietitians. Clinical pharmacists, quality outcomes directors, and clinical investigators will find the document useful. We write primarily for practitioners in North America. Nevertheless, from the onset, we have coordinated our efforts with guideline development processes elsewhere, ensured that Work Group membership includes experts from Latin America and Europe, and planned so that this document may serve as the last of the KDOQI Anemia Guidelines and a foundation for the first truly global guideline under the auspices of the Kidney Disease: Improving Global Outcomes (KDIGO) initiative.
Scope
We address the target population of patients with CKD stages 1 to 5 not on dialysis therapy, on hemodialysis (HD) or peritoneal dialysis (PD) therapy, or with a kidney transplant in the full range of practice settings in which they are encountered. However, the evidence continues to derive disproportionately from findings in facility-based HD patients. We have not been unmindful of cost implications. However, net medical benefit to the patient is the foundation of each of our guidelines and recommendations. Our commitment, in short, is to assist practitioners in the care of patients by describing best practice and the evidence for it. In this way, individual practitioners who face local reimbursement constraints may make informed decisions armed with appropriate facts. Similarly armed, those responsible for guiding reimbursement policy may make informed decisions consistent with the evidence.
Evidence, Opinion, Guidelines, and Recommendations
When the quality of evidence is high or moderately high, we present a CPG based on evidence, rate the quality of that evidence, and distinguish the evidence-based guideline by enclosing it in a text box. When the quality of evidence is low, very low, or missing, but the topic is important to practitioners, we offer a CPR and cite limitations to the current literature. In a subsequent document, we will propose and prioritize needed research. Throughout this document, a text box surrounds each evidence-based guideline, and the strength of the guideline is rated as “strong” or “moderately strong.” The phrase, “In the opinion of Work Group members” precedes each CPR. Distinguishing evidence-based guidelines from CPRs is in keeping with recent advances in KDOQI policy and practice.
The overall strength of each guideline statement was rated by assignment as either strong or moderately strong. A strong rating indicates “it is strongly recommended that clinicians routinely follow the guideline for eligible patients. There is high-quality evidence that the practice results in net medical benefit to the patient.” The moderately strong rating indicates “it is recommended that clinicians routinely follow the guideline for eligible patients. There is at least moderately high-quality evidence that the practice results in net medical benefit to the patient.” Overall, the strength of the guidelines and recommendations was based on the extent to which the Work Group could be confident that adherence will do more good than harm. Strong guidelines require support by evidence of high quality. Moderately strong guidelines require support by evidence of at least moderately high quality. Incorporation of additional considerations modified the linkage between quality of evidence and strength of guidelines, usually resulting in a lower strength of the recommendation than would be supportable based on the quality of evidence alone.
Our objective is to describe the evidence base for key elements in the identification, evaluation, and management of patients with CKD-associated anemia. For topics on which we undertook a systematic literature review, we present detailed information, usually in the form of summary evidence tables. These topics include Hb thresholds for initiating therapy, Hb level therapeutic goals, iron status goals, or efficacy of adjuvants in achieving Hb goals. Results that bear directly on patient lives (mortality, morbidity, QOL, and adverse events [AEs]) receive particular attention. Topics for which the evidence base is limited deserve brief mention and receive it. Information that involves implementation, application, or protocol development, we leave to the NKF Kidney Learning System (KLS) resources.
When the quality of evidence is low, we will follow acknowledgement of these limitations in a separate publication by encouraging further investigation.
Relationship to Previous Guidelines
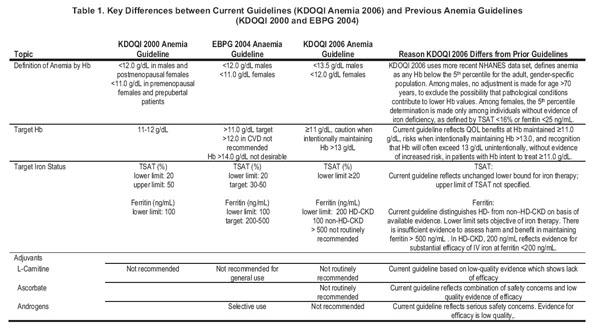
The lineage of the current document derives from both the most recent (2001) version of the KDOQI Guidelines for Anemia2 and the most recent (2004) version of the European Best Practices Guidelines (EBPGs) for anemia (Fig 2). 16 Our intention is to simplify the current guidelines into sections on identification, definition, and evaluation of anemia (Guideline 1.1 and 1.2); Hb treatment target (Guideline 2.1); and management (Guidelines 3.1-4.1), with parallel organization for pediatrics. Key differences between current and past guidelines are set out in Table 1.
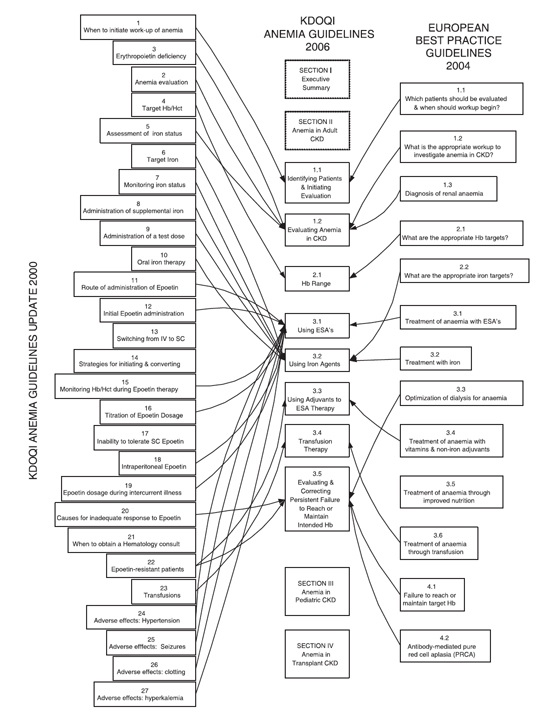
Fig 2. Relationship between current and previous anemia guidelines.
OPERATING DEFINITIONS
Erythropoiesis-stimulating agent (ESA): The term ESA applies to all agents that augment erythropoiesis through direct or indirect action on the erythropoietin receptor. Currently available ESAs include epoetin alfa, epoetin beta, and darbepoetin alfa.
Therapeutic goal: A therapeutic goal is the intended goal of current therapy, not the current result of previous therapy. This definition applies equally to therapeutic goals for both Hb and iron.
Indicators of Efficacy:
Efficacy of anemia management: Efficacy of anemia management is indicated in a single patient by an Hb level greater than the target threshold, and in a group of patients, by the percentage of patients maintained at greater than target threshold.
ESA efficacy: ESA efficacy is indicated by the dose of ESA needed to achieve or maintain Hb levels at greater than the target threshold.
Adjuvant efficacy: Adjuvant efficacy is indicated by the percentage of patients who successfully exceed the target Hb threshold or achieve an increase in Hb level greater than 1 g/dL without initiating ESA therapy or increasing ESA doses to greater than baseline. Among patients with steady-state Hb levels greater than target threshold, adjuvant efficacy is indicated by ESA dose reduction.


