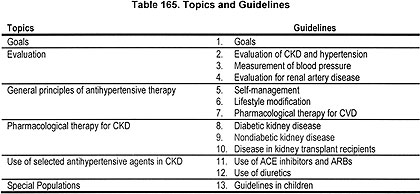
THE OVERALL AIM of the project was to develop evidence-based recommendations for blood pressure management in individuals with CKD. The Work Group sought to develop an "evidence base" for the recommendations that is derived from a systematic review of the available scientific literature on the evaluation of blood pressure in individuals with CKD, measurement of blood pressure, treatment with lifestyle modifications, and treatment with pharmacological therapy, including selection of antihypertensive agents and treatment targets. Topics considered are listed in Table 165. As a result, this report consists of a set of clinical practice guidelines and the summary tables presenting the evidence on which the guidelines are based.
Definitions
Table 166 gives a list of definitions used in the guidelines. In addition, the Work Group developed the following operational definitions for these guidelines:
Antihypertensive therapy includes lifestyle modifications and pharmacological therapy that lower blood pressure, in patients with or without hypertension.
Lifestyle modifications include changes in diet, exercise and habits that may lower the risks of progression of CKD and CVD. These guidelines focus specifically on lifestyle modifications that lower blood pressure. Lifestyle modifications are discussed in more detail in Guideline 6.
Pharmacological therapy includes selection of antihypertensive agents and blood pressure goals. General principles of pharmacological therapy and target blood pressure for CVD risk reduction are discussed in Guideline 7.
Antihypertensive agents are defined as agents that are usually prescribed to lower blood pressure. Other agents may also lower blood pressure as a side-effect. Many antihypertensive agents have effects in addition to lowering systemic blood pressure and are used for indications other than hypertension.
"Preferred agents" are classes of antihypertensive agents that have beneficial effects on progression of CKD or reducing CVD risk, in addition to their antihypertensive effects, such as reducing proteinuria, slowing GFR decline, and inhibiting other pathogenetic mechanisms of kidney disease progression and CVD. In certain types of CKD, specific classes of antihypertensive agents, notably those that inhibit the RAS, are preferred agents for slowing progression of CKD. Thus, the Work Group developed guidelines that recommend the use of specific classes of antihypertensive agents in certain types of CKD, even if hypertension is not present.
Target Population
Based on the results of clinical and epidemiological studies, the Work Group defined the target population for these guidelines as adults or children with CKD Stages 1-4. CKD is defined as kidney damage (abnormalities on biopsy, urinalysis, abnormal imaging studies) and/or GFR <60 mL/min/1.73 m2 and includes the following causes of CKD: diabetic kidney disease, glomerular diseases, nephrosclerosis, tubulointerstitial diseases, cystic diseases, diseases in the kidney transplant, and renal artery disease. Stages 1-4 correspond to a GFR ≥15 mL/min/1.73 m2. Individuals on dialysis or those with a GFR <15 mL/min/1.73 m2 were excluded based on the different pathophysiology and treatment modalities applicable to them. Kidney transplant recipients with CKD Stages 1-4 were included.
Patients with CKD Stage 5 (kidney failure) were excluded for several following reasons. First, kidney disease progression may not be as important in patients who have already reached the stage of kidney failure. Second, the relationship between CVD risk and level of blood pressure is complex in kidney failure. Third, blood pressure in hemodialysis patients is affected by intermittent fluid gains and fluid removal. The Work Group has included recommendations for both adults and children. Guideline 13 for children was written with careful consideration to past recommendations for the treatment of hypertension in children as noted in JNC 615 and from the National High Blood Pressure Education Program Work Group for children and adolescents.10 Figure 62 shows the evolution of National Kidney Foundation guidelines for the management of hypertension in patients with CKD.
Fig 62. The evolution of National Kidney Foundation Guidelines on Hypertension and Antihypertensive Agents in CKD.
Intended Users
These guidelines are intended for use by physicians, nurse practitioners, registered nurses, registered dietitians, masters prepared social workers, pharmacists, physician assistants, and other professionals caring for patients with CKD. The information contained in these guidelines can and should be conveyed to patients and their families in an understandable manner by their physician and/or other health-care professionals. The development of educational support materials designed specifically for patients and their families should be part of the implementation of these guidelines.
Anticipated Updates
All guidelines should be updated whenever new, pertinent information becomes available. To anticipate when these guidelines may need to be updated, the Work Group discussed ongoing, controlled trials in the general population and in patients with CKD, as those results may be pertinent to some recommendations (Table 167). Given the potential for these and other studies to provide information relevant to the assessment and treatment of hypertension in patients with CKD, it was concluded that these guidelines should be updated in about 3 to 4 years from the time of publication, and sooner if new, pertinent information becomes available before then. The Work Group will monitor the progress of these trials and recommend updating these guidelines as indicated.
KDOQI Principles and Process
The development of these guidelines followed four basic principles set forth by KDOQI (Table 168).
The guidelines were developed using an evidence-based approach similar to that endorsed by the Agency for Healthcare Research and Quality. Development of the guideline and evidence report required many concurrent steps (Table 169). The Work Group reviewed all pertinent, published evidence, and critically appraised the quality of studies and the overall strength of evidence supporting each recommendation.
Creation of Work Group and Evidence Review Team
The Work Group was convened by the NKF Kidney Disease Outcomes Quality Initiative (KDOQI) in response to recommendations of the NKF Task Force on CVD in Chronic Renal Disease.3 The Co-Chairs of the KDOQI Advisory Board selected the Work Group Chair and Director of the Evidence Review Team, who then assembled groups responsible for the development of the guidelines and the evidence report, respectively. The Work Group and Evidence Review Team first met in August 2000. Both groups collaborated closely throughout the project.
The Work Group consisted of "domain experts," including individuals with expertise in adult and pediatric nephrology, cardiology, epidemiology, nutrition, social work, and family medicine. In addition, the Work Group had liaison members from the National Institute of Diabetes, Digestive and Kidney Diseases, from the National Heart Lung and Blood Institute and from the Renal Physicians Association. The Work Group also maintained communication with members of the JNC 75 and ADA 20036 and with investigators of the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)107 for which results were announced during the time the Work Groups held meetings.
The first task of the Work Group chair and the members was to define the overall goals and topics, including specifying the target condition, target population, and target audience. The domain experts then assisted the evidence review team in further refining topics, developing the literature search strategy, and drafting data extraction forms. The Work Group members were the principal reviewers of the literature. They used the evidence compiled in summary tables as a basis for the guidelines and took the lead in writing the guidelines and rationale statements.
The Evidence Review Team consisted of nephrologists and methodologists from Tufts-New England Medical Center and elsewhere with expertise in systematic review of the medical literature. They were responsible for seeing to project timelines and compiling of the evidence report. Specific tasks included refining the questions to be addressed, developing literature search strategies, establishing inclusion and exclusion criteria, running searches, screening of abstracts and articles, verification of data extraction, compilation of evidence and summary tables, consultation on all aspects of methodology and process, coordination and tracking of project tasks. Throughout the project the Evidence Review Team led discussions on the methodology of systematic review, literature searches, data extraction, assessment of quality of articles, summary reporting, formulation of guidelines statements and rationale text, and grading of the strength of guideline recommendations and rationale statements.
Development of Topics
The goals of the Work Group spanned the following topics: (1) evaluation of individuals with CKD or hypertension, (2) measurement of blood pressure including ambulatory blood pressure monitoring (ABPM), (3) evaluation of renal artery disease (RAD), (4) treatment of hypertension with lifestyle modifications, and (5) use of antihypertensive agents, including selection of antihypertensive agents and blood pressure targets, to slow progression of kidney disease and reduce CVD risk in CKD.
Refinement of Topics and Development of Materials
The Work Group and Evidence Review Team developed (1) draft guideline statements; (2) draft rationale statements that projected the pertinent evidence; (3) mock summary tables providing a shell for the expected evidence; and (4) data extraction forms prompting the reviewers for relevant data elements to be retrieved from the primary articles. The development process included creation of initial mock guidelines and tables by the Work Group Chair and Evidence Review Team, followed by iterative refinement by the Work Group members. The refinement process began prior to literature retrieval and continued through all stages of the project until the drafting of the final report. The refinement occurred by e-mail, telephone, and in-person communication regularly with local experts and with all experts during in-person meetings of the Evidence Review Team and Work Group members. Throughout the process of topic refinement, the type of study design that would be appropriate to address the topics of interest was carefully considered.
Data extraction forms were designed to capture information on various aspects of the primary articles. Data extraction included objective information such as study setting and patient demographics, eligibility criteria, causes of kidney disease, numbers of subjects, study design, study funding source, definitions of measures, interventions in controlled trials and outcomes (mortality, CVD, kidney, and other outcomes as well as adverse events). Work Group members were also asked to provide their assessments of applicability of the study population (see below), methodological quality of the study (based on criteria appropriate for each study design, see below), appropriateness of the selected measures, and assessment of biases and other comments.
The Evidence Review Team conducted training sessions for the Work Group members to learn systematic data extraction from primary articles. Evidence Review Team reviewed and verified the data forms completed by Work Group members. Feedback was provided to individual members and discrepancies were reconciled by e-mail and teleconferences.
Review of Existing Guidelines on CKD
There were several prior guideline recommendations for blood pressure management in CKD, as well as other documents that became available during the course of guideline development and these were reviewed by the Work Group. The recommendations issued in 1997 by the Sixth Joint National Commission (JNC 6) were considered a reference source.15 Thus, the search for other guidelines with recommendations on blood pressure management in CKD was restricted to guidelines published since 1997. Searches were conducted through the National Guideline Clearing House on the following keywords: hypertension, kidney disease, diabetes, and cardiovascular disease, spanning the time from 1997 until March 2003. Approximately 150 guideline citations were found. All guidelines that could be downloaded free of charge or retrieved from published medical journals were searched for recommendations for blood pressure management and use of antihypertensive agents in CKD. Through this search, we found 11 guidelines with pertinent sections and supplemented this with other known recommendations (Table 170). The relevant recommendations of these other guidelines are reviewed in this guideline document in Guidelines 8, 9, 10, and 13.
Review of Existing Guidelines on CVD in the General Population
Because we found few studies that examined the effect of pharmacological antihypertensive therapy on CVD outcomes in CKD, the Work Group decided to extrapolate the results from studies conducted in the general population and in other, high-risk populations to those with CKD. Some would argue that no guideline statements should be made in the absence of evidence on clinical outcomes in the target population. However, the limited number of studies on antihypertensive therapy in CKD with CVD outcomes, compared to the large number of studies in the general population, required development of criteria for extrapolating evidence from the general population to the target population. Table 171 compares evidence derived from studies in the general population to evidence derived from studies in patients with CKD.
Based on this comparison, the Work Group adopted the criteria developed by the NKF Task Force on Cardiovascular Disease in Chronic Renal Disease for extrapolating evidence collected in trials studying cardiovascular outcomes, mortality, or total mortality from the general population to patients with CKD.
1. The mechanisms and expression of CVD in CKD should be similar to those observed in the general population. Specifically, the features of CVD, the relationship of the risk factor (hypertension) to CVD outcomes, the mechanism of risk factor reduction (blood pressure lowering), and the responsiveness of the risk factor to therapies (lifestyle modifications and pharmacological therapy) should be similar in patients with CKD and in the general population.
2. Therapies in patients with CKD should be as safe, or nearly so, as in the general population. In particular, there should not be additional adverse effects of a specific therapy that limits its usefulness in patients with CKD, either because of altered pharmacokinetics, drug interactions, or increased risk of toxicity to the kidney.
3. The duration of therapy required to improve CVD outcomes in the general population should not exceed the life expectancy of patients with CKD. In other words, it should not already be too late to intervene in this generally elderly, sick, and frail population. Determining whether patients with CKD can survive for long enough to gain the benefit of therapy for CVD is a difficult question. Numerous studies show a dramatically shortened life expectancy for patients with CKD, especially patients with kidney failure. For example, the USRDS395 has estimated that the average life expectancy of 60- to 64-year-old patients treated by dialysis ranges from 3.6 to 5.1 years, depending on gender and race. On the other hand, the most common cause of death in kidney failure is CVD, and numerous studies of CVD in the general population have shown a benefit of interventions within 2 to 5 years, with greater and earlier benefits in patients at highest risk. Thus, it is likely that patients with kidney failure could benefit from more effective treatment of CVD. Because of their longer life expectancy, patients with earlier stages of CKD might be most likely to benefit.
However, a systematic review of all the pharmacological blood pressure trials with CVD outcomes in the general population was felt to be beyond the scope or the expertise of the Work Group. Therefore, it was decided to review existing guidelines for recommendations on blood pressure targets and specific antihypertensive agents for prevention of CVD. The same search that was conducted through the National Guideline Clearinghouse to identify existing guidelines with recommendations on managing blood pressure in CKD (see above) was also screened for sections containing recommendations on risk reduction for CVD using antihypertensive agents (Table 172). This yielded 13 guidelines which were abstracted and compiled into tables and reviewed by Work Group experts. The relevant recommendations are reviewed in Guideline 7.
Literature Search of Primary Articles
The Work Group and Evidence Review Team decided in advance that a systematic process would be followed to identify primary studies on the topics of interest. Only fully published articles with original data were included. Review articles, editorials, letters or abstracts were generally excluded. The only exception were abstracts of the ALLHAT trial reporting kidney disease progression and CVD outcomes in a large subgroup of individuals with CKD.292,293 The Work Group members and Evidence Review Team selected textbooks and review articles based on personal knowledge.
Studies for the literature review were identified primarily through Medline searches of the English language literature. The Medline literature searches were conducted between July 2001 and July 2002 to identify clinical studies published from 1966 through the search dates. Separate search strategies were developed for each topic.
Development of the search strategies was an iterative process that included input from all members of the Work Group. The text words or medical subject headings (MeSH) included kidney or kidney diseases or kidney function tests or hypertension or renal, diabetic nephropathy, renal artery, and ambulatory blood pressure monitoring. The searches were limited to human studies. Studies that focused on hemodialysis or peritoneal dialysis, pregnancy, neonates, malignant hypertension, acute renal failure, or pharmacokinetics were excluded.
The Evidence Review Team screened citations identified by the Medline search. Potentially relevant articles were identified from abstracts and titles, based on study population, relevance to specific guideline topics, and the study design. In general, studies with fewer than 10 subjects per treatment arm were excluded. However, for pediatric topics, due to the small number of articles, only case reports were excluded. Relevant articles known to domain experts and reviewers supplemented these searches. After retrieval, each paper was screened according to the established criteria to verify its relevance and appropriateness. Work Group members assigned to the specific topic reviewed the articles and made the final decision for inclusion or exclusion of articles. However, they had to provide the reason for rejection. Data extraction was performed on all included articles and their data compiled into evidence tables. Additional relevant studies published since July 2002 were added by experts.
Table 173 lists the details of the literature search and review for each topic. Overall, 11,688 abstracts were screened by the Evidence Review Team, 899 articles were retrieved and reviewed, and data were extracted from 177 articles. Forty-seven articles were added by the Work Group. Finally, results from 76 articles were systematically included in the summary tables.
The Medline search strategies are shown in Appendix 2.
Study Selection
Evaluation and blood pressure measurement. Literature review did not yield any controlled trials of adequate quality or study size in individuals with CKD on the topics of evaluation, blood pressure measurement (with the exception of studies on ABPM), and lifestyle modifications as treatment for blood pressure. For these topics, we used existing guidelines.
ABPM in CKD. The Work Group felt that a detailed discussion on technical aspects of ABPM and its use in the management of blood pressure in CKD was important. However, since the evidence was not strong enough to recommend routine use of ABPM in CKD at the present time, a detailed review of this topic was included as a technical appendix. The issues the Work Group had identified to address regarding the use of ABPM in CKD were (1) correlation of office blood pressure with ambulatory blood pressure (ABP); (2) prevalence of white coat hypertension (WCH); (3) prevalence of abnormal blood pressure patterns; (4) association between ABP and kidney or CVD outcomes; (5) risk relationship of ABPM with subsequent clinical outcomes; and (6) impact of ABPM on blood pressure control or antihypertensive therapy. The search strategy for ABPM is shown in Appendix 2.
During the course of the guideline development process, an evidence report on "Utility of Blood Pressure Monitoring Outside of the Clinic Setting" commissioned by the Agency of Healthcare Research and Quality (AHRQ) became available.142 This report had systematically synthesized the available evidence on ABPM addressing the questions our Work Group had drafted and using very similar screening criteria. In addition to monitoring of blood pressure by ABPM, the AHRQ report had also examined the utility of self-measured blood pressure. The inclusion criteria of the AHRQ report were not restricted to, but included, studies conducted in the target population of adults or children with CKD. Thus, the Work Group felt that duplication of effort was not warranted and formal data abstraction of the articles identified by the search or synthesis of results into summary tables was not pursued. Rather, it was decided to incorporate the findings of the AHRQ report into this guideline and to cite the previously retrieved studies in a narrative format.
Renal Artery Disease (RAD). For the topic of evaluation of individuals with CKD for RAD, we examined the literature for meta-analyses comparing the accuracy of diagnostic tests for RAD. This yielded one relevant study, which was reviewed according to the criteria in Tables 174 and 175. Another study that contains a clinical prediction model was added by the Work Group to estimate the probability of RAD.
Nonpharmacological therapies. We searched for nonpharmacological therapy trials such as lifestyle modifications with diet, exercise, and weight reduction that were conducted to lower blood pressure and thereby reduce the risk for kidney or heart outcomes. We did not find any studies with a randomized or nonrandomized controlled design that had at least 10 CKD subjects per treatment arm and reported outcomes of interest.
Pharmacological therapy with selected antihypertensive agents or blood pressure treatment targets. For pharmacological therapy, we examined RCTs using antihypertensive agents or blood pressure modifying therapies. We reviewed studies providing comparisons on the two interventions of interest: use of selected antihypertensive agents or achievement of predefined blood pressure targets (Table 176). Outcomes of interest were kidney outcomes, cardiovascular outcomes, and total mortality and adverse effects.
For evaluation of antihypertensive agents, we reviewed RCTs in patients with CKD with or without elevated blood pressure for secondary prevention. For example, we included studies of patients with the earliest stage of CKD due to diabetes (microalbuminuria), regardless of blood pressure at baseline (including normotensive patients). On the other hand, we excluded studies of patients with diabetes without kidney disease, even if hypertensive, thereby excluding primary prevention studies in diabetes.
For evaluation of the level of blood pressure, we reviewed RCTs comparing the effect of different predefined blood pressure ranges that were targeted with a combination of antihypertensive agents. Since the number of RCTs using predefined blood pressure targets was small, we also screened prospective cohort studies that reported risks of outcomes stratified by blood pressure levels. We were interested in data that allowed us to look at rates of events stratified by different blood pressure levels to discover nonlinear relationships between blood pressure level and risk for outcomes and to investigate threshold relationships in order to help define optimal blood pressure levels. However, since these results were reported in many different ways, they were difficult to compare across studies and did not allow us to answer the question of a threshold relationship with confidence. Thus, observational studies were not included. Instead, the recommendations for blood pressure targets were derived from the review of existing guidelines that built on the knowledge of a positive correlation between blood pressure level and CVD risk and the evidence that reduction of blood pressure reduced CVD risk.
In addition, effect modifiers such as demographic or genetic factors were searched for as a routine part of the data abstraction of each treatment study.
The questions of preferred agents and optimal blood pressure targets were posed for all types of kidney disease. Given the paucity of studies in kidney transplant recipients, we lowered the criteria. For kidney disease in the kidney transplant and outcome proteinuria, we accepted n ≥ 10 per arm (instead of n ≥50/arm) and follow-up ≥1 months (instead of ≥3 months) (Table 176).
For RAD, screening yielded a limited number of studies that were not standardized very well, with overall short follow-up. The Work Group deemed this body of evidence to be insufficient to recommend selection of pharmacological agents or blood pressure targets. In addition, the Work Group decided that giving recommendations on how to treat individuals with RAD with other treatment modalities was beyond the scope of this project. This decision was based on the recognition that interventional therapy of renal artery disease is a field in flux because of the advent and testing of rapidly evolving novel interventional devices and techniques.
In children, the searches yielded only few studies with small numbers and short follow-up. These studies are referred to in narrative form as appropriate rather than summarized in summary table format.
Adverse events of preferred agents. The objective of this topic was to characterize the safety and tolerance of ACE inhibitors or ARBs in individuals with CKD. The rationale was that these agents would be recommended as preferred agents for a large number of individuals who have certain types of CKD. We wanted to address the concern that these agents can cause acute loss of kidney function or hyperkalemia, especially in individuals with decreased GFR. Thus, we collected data on the frequency and severity of adverse events from ACE inhibitors or ARBs, specifically, "early decrease of GFR" and hyperkalemia. We used RCTs, non-RCTs, prospective or retrospective cohort studies, and case-control studies in all types of CKD including RAD.
Format for Evidence Tables
Two types of tables were prepared using data extracted from accepted articles. Evidence tables contain data derived from the data extraction forms that covered features of the study design, patient demographics, disease characteristics, interventions, definitions of the outcomes and their results. These detailed evidence tables, with information spanning across several pages for each study, were made available to Work Group members for the purpose of reviewing the data and writing of guidelines. The evidence tables are not published along with the guidelines.
Using the information in the evidence tables, the Evidence Review Team also prepared a set of summary tables that succinctly describe the characteristics for each study in six areas: study size; applicability (type of study subjects); baseline information (kidney function, level of proteinuria, and blood pressure in comparator group one); information on therapy in each treatment arm (antihypertensive agents or blood pressure targets tested as well as the blood pressure at the end of the study); results of the primary outcome (the main or composite primary outcome and the magnitude of the effect); and methodological quality.
For outcomes, only the primary outcome is represented. Composite outcomes that were primarily kidney outcomes (eg, doubling of creatinine and ESRD) but also contained all-cause mortality were coded as kidney outcomes. Similarly, composite outcomes combining outcomes related to kidney function and proteinuria were summarized under kidney outcomes. Thus, the outcome for proteinuria is only coded in the proteinuria column for studies where proteinuria was the sole primary outcome.
Within each of the summary tables, studies were ordered first by methodological quality (highest to lowest), then by applicability (most to least), and then by study size (largest to smallest). In summary tables reporting side effects, studies are ordered first by type of disease (diabetic kidney diseases first), then by study design (randomized controlled trials first), then by study size (largest first).
Summary tables are presented in the guideline section that corresponds to the respective topic. An example of a summary table is shown in Table 177.
Study Size
The number of patients (N, sample size) is used as a measure of the weight of the evidence. In general, large studies provide more precise estimates of effects or associations. In addition, results from large studies are more likely to be applied to a broader population; however, large size alone does not guarantee a high degree of applicability. A study that enrolls a large number of selected patients may be less generalizable than several smaller studies that include a broad spectrum of patient populations.
Applicability
Applicability (also known as generalizability or external validity) addresses the issue of whether the study sample is sufficiently broad so that the results can be applied to the population of interest at large. The study sample is defined by the inclusion and exclusion criteria. The target population was defined to include patients with CKD and those at increased risk of CKD, except where noted. A designation for applicability was assigned to each article, according to a three-level scale. In making this assessment, sociodemographic characteristics were considered, as were the stated causes of CKD and prior treatments. If the study is not considered broadly generalizable, reasons for the limited applicability are reported. Table 178 describes our approach to assessing applicability.
Results
The type of results available in each study is determined by the study design, the purpose, and the question(s) being asked. The Work Group decided that the outcomes of interest were primary outcomes documenting effects on kidney disease progression, all-cause mortality, or cardiac outcomes in RCTs. Given the large spectrum of different outcomes, results were collapsed into four categories: direct health outcomes related to kidney disease progression (kidney failure, changes in GFR, changes in serum creatinine), surrogate outcome category related to kidney disease progression (proteinuria), direct health outcomes related to CVD (clinical events), and surrogate outcomes related to CVD (LVH or carotid artery intima thickness). Table 179 shows our approach in reporting comparisons of blood pressure targets or antihypertensive agents. For studies reporting adverse effects of ACE inhibitors and ARBs, the incidence of adverse effects (ie, drug reduction or discontinuation for hyperkalemia, for early decrease in GFR or for any adverse effect) were reported as percentages.
Methodological Quality
Methodological quality (or internal validity) refers to the design, conduct, and reporting of the clinical study. Because studies with a variety of types of design were evaluated, a generic three-level classification of study quality was devised (Table 180).
Summarizing Review Articles and Selected Original Articles
Work Group members had wide latitude in summarizing review articles. They selected original articles for topics that were determined, a priori, not to require a systematic review of the literature. The use of published or derived tables and figures was encouraged to simplify the presentation.
Guideline Format
This document contains 13 guidelines. The format for each guideline is outlined in Table 181. Each guideline contains one or more specific, numbered "recommendations" or "guideline statements." Each guideline contains background information, which is generally sufficient to interpret the guideline. The rationale for each guideline contains definitions, if appropriate, and a section on the strength of evidence. The strength of evidence includes a series of specific "rationale statements," each supported by evidence, and "summary tables" (if appropriate) compiling and evaluating original reports of studies. The guideline concludes with a discussion of limitations of the evidence review and a brief discussion of clinical applications, implementation issues, and research recommendations regarding the topic.
Rating the Strength of Guidelines and Rationale Statements
The overall strength of each guideline statement was rated by assigning either "A," "B," or "C" (Table 182). An "A" rating indicates "it is strongly recommended that clinicians routinely follow the guideline for eligible patients. There is strong evidence that the practice improves health outcomes, and benefits substantially outweigh harms." The "B" rating indicates "it is recommended that clinicians routinely follow the guideline for eligible patients. There is moderate evidence that the practice improves health outcomes." A "C" rating indicates "it is recommended that clinicians consider following the guideline for eligible patients. This recommendation is based on either weak evidence, or on the opinions of the Work Group and reviewers, that the practice might improve health outcomes."
The strength of evidence was graded using a rating system that takes into account: (1) methodological quality of the studies; (2) whether or not the study was carried out in the target population, ie, patients with CKD, or in other populations; and (3) whether the studies examined health outcomes directly, or examined surrogate measures for those outcomes, eg, reducing proteinuria rather than slowing progression of CKD (Table 183). These three separate study characteristics were combined in rating the strength of a body of evidence provided by the composite of the pertinent studies.
In addition, the Work Group adopted a convention for using existing expert guidelines issued for populations other than the target population. Grades assigned by the guideline-issuing bodies for the strength of evidence were adopted. When the guideline or the evidence were not graded, this Work Group assumed that the guideline would be based on at least moderately strong evidence. The extrapolation of ungraded guideline recommendations from the general populations to the target population was considered to support grade B recommendations.
Limitations of Approach
While the literature searches were intended to be comprehensive, they were not exhaustive. Medline was the only database searched, and searches were limited to English language publications. Hand searches of journals were not performed, and review articles and textbook chapters were not systematically searched. Important studies known to the domain experts and reviewers that were missed by the literature search were included in the review.
Due to the wide variety of methods of analysis, units of measure, definitions of CKD, and methods of reporting in the original studies, it was often very difficult to standardize the findings for this report.