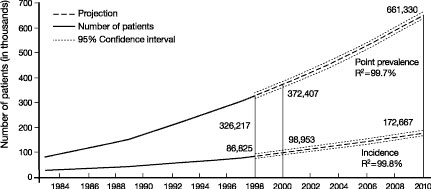
CHRONIC KIDNEY disease (CKD) is a worldwide public health issue. In the United States, there is a rising incidence and prevalence of kidney failure (Fig 1), with poor outcomes and high cost.2 The prevalence of earlier stages of CKD is approximately 100 times greater than the prevalence of kidney failure, affecting almost 11% of adults in the United States.1 There is growing evidence that some of the adverse outcomes of CKD can be prevented or delayed by preventive measures, early detection, and treatment.
Fig 1. Kidney failure in the United States. Incidence and prevalence of kidney failure treated by dialysis and transplantation (end-stage renal disease) in the United States. Incident patients refers to new cases during the year. Point prevalent patients refers to patient alive on December 31st of the year. Solid vertical lines represent complete data for 1998 and expected data for 2000. Projections for future years are based on extrapolation of regression equations. R2 for regression equations is given. From USRDS 2000 Annual Data Report.2
Hypertension is a cause and complication of CKD. Hypertension in CKD increases the risk of important adverse outcomes, including loss of kidney function and kidney failure, early development and accelerated progression of cardiovascular disease (CVD), and premature death.3,4 In the ongoing effort to improve outcomes of CKD, the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (K/DOQI) appointed a Work Group and an Evidence Review Team in 2001 to develop clinical practice guidelines on hypertension and use of antihypertensive agents in CKD. During this same time, clinical practice guidelines on this topic relevant to CKD were also under development by other organizations, including the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)5,5a and the 2003 report of the American Diabetes Association (ADA) on the Treatment of Hypertension in Adults with Diabetes.6 The Work Group maintained contact with these organizations during development of these guidelines.
The purpose of the Executive Summary is to provide a "stand-alone" summary of the background, scope, methods, and key recommendations, as well as the complete text of the guideline statements. Most tables and figures in the Executive Summary are taken from other sections of the document.
Chronic Kidney Disease
Figure 2 is a conceptual model of CKD, which defines stages of CKD, as well as antecedent conditions, outcomes, risk factors for adverse outcomes, and actions to improve outcomes.
Fig 2. Conceptual model for stages in the initiation and progression of CKD and therapeutic interventions. Shaded ellipses represent stages of CKD; unshaded ellipses represent potential antecedents or consequences of CKD. Thick arrows between ellipses represent "risk factors" associated with initiation and progression of disease that can be affected or detected by interventions: susceptibility factors (black); initiation factors (dark gray); progression factors (light gray); and end-stage factors (white). Interventions for each stage are given beneath the stage. Individuals who appear normal should be screened for CKD risk factors. Individuals known to be at increased risk for CKD should be screened for CKD. Complications refer to all complications of CKD and its treatment, including complications of decreased GFR (hypertension, anemia, malnutrition, bone disease, neuropathy, and decreased quality of life) and cardiovascular disease. Reprinted with permission.1 Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate.
CKD is defined as kidney damage, as confirmed by kidney biopsy or markers of damage, or glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for ≥ 3 months (Table 1).1 Markers of kidney damage include proteinuria, abnormalities on the urine dipstick or sediment examination, or abnormalities on imaging studies of the kidneys. GFR can be estimated from prediction equations based on serum creatinine and other variables, including age, sex, race, and body size.
Among individuals with CKD, the stage of disease is based on the level of GFR (Table 2), irrespective of the cause of kidney disease.1 The high prevalence of earlier stages of CKD emphasizes the importance for all health-care providers, not just kidney disease specialists, to detect, evaluate, and treat CKD.
Hypertension in CKD
JNC 7 defines hypertension as systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg, respectively (Table 3).5
Although common in CKD, hypertension is not part of the definition of CKD. Table 4 illustrates the classification of individuals based on presence or absence of kidney damage and hypertension, and level of GFR. Approximately 50% to 75% of individuals with GFR <60 mL/min/1.73 m2 (CKD Stages 3-5) have hypertension (Fig 3).1 Among individuals with GFR ≥ 60 mL/min/1.73 m2, distinguishing CKD Stages 1 and 2 (Table 4, shaded areas) from "hypertension" and "hypertension with decreased GFR" (Table 4, unshaded areas) requires assessment for markers of kidney damage. This is especially important in the elderly, in whom both hypertension and decreased GFR are common.
Fig 3. Prevalence of high blood pressure by level of GFR, adjusted to age 60 years (NHANES III). GFR was estimated using the abbreviated MDRD Study equation. Hypertension was defined as JNC ≥ Stage 1 (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or taking medications for hypertension) or JNC ≥ Stage 2 (SBP ≥ 160 or DBP ≥ 100 mm Hg). Values are adjusted to age 60 years using a polynomial regression. 95% confidence intervals are shown at selected levels of estimated GFR. Reproduced with permission.1
Cardiovascular Disease in CKD
CKD is a risk factor for cardiovascular disease (CVD).3,4,7 Dialysis patients have a 50 to 500 times increased risk of CVD mortality compared to age-matched individuals from the general population (Fig 4). Earlier stages of CKD are also associated with an increased risk of CVD. CKD is associated with an increased prevalence and severity of both "traditional" and "nontraditional" risk factors for CVD. Traditional risk factors include those initially described in the Framingham Study. Among traditional risk factors, hypertension is closely linked to CKD and has often been implicated as the main cause of CVD in CKD. Other traditional risk factors for CVD that are common in CKD include older age, diabetes and hyperlipidemia. Nontraditional risk factors for CVD such as inflammation, malnutrition, mineral disorders (calcium and phosphorus), and anemia are also common in CKD. In addition, albuminuria (Fig 5)8 and decreased GFR (Fig 6)9 are associated with an increased risk of CVD, even after controlling for many of these risk factors. Early detection and treatment of CKD, including detection and treatment of hypertension and other CVD risk factors, may reduce the risk of CVD in CKD. Achieving these goals in CKD will require coordinating antihypertensive therapy with therapy for other CVD risk factors.
Fig 4. CVD mortality in dialysis patients (USRDS) compared to the general population (NCHS). CVD mortality defined by death due to arrhythmias, cardiomyopathy, cardiac arrest, myocardial infarction atherosclerotic heart disease, and pulmonary edema in the general population (GP), data from National Center for Health Statistics (NCHS) multiple cause of mortality data files (ICD 9 codes 402, 404, 410-414, and 425-429, 1993) compared to dialysis patients (data from USRDS special data request). HCFA form 2746, #s 23, 26-29, and 31, 1994-1996. Data are stratified by age, sex, and race. Reproduced with permission.24
Fig 5. Albuminuria as a risk factor for CVD. The adjusted effect of urinary albumin concentration (UAC) on hazard function in the Prevention of Renal and Vascular End Stage Disease (PREVEND) Study. The solid line shows the estimated relationship when logarithmic hazard is modeled as a linear function of log[UAC]. The dotted lines are 95% confidence limits for a more general functional relationship, as estimated by P-splines. The hatched area represents UAC of 20 to 200 mg/L, respectively, corresponding approximately to the definition of microalbuminuria. The graphs show that as UAC increases, the hazard ratios for both cardiovascular and noncardiovascular death increases. This increase begins in individuals with UAC in the microalbuminuria range. Reproduced with permission.8
Fig 6. Decreased GFR as a risk factor for CVD. Five-year probability of CVD events according to baseline estimated GFR, as observed in 45- to 64-year-old individuals enrolled in the Atherosclerosis Risk in Communities (ARIC) Study. Hatch marks on the horizontal axis indicate number of individuals with events at corresponding level of GFR. The large increase in risk for individuals with baseline GFR <60 mL/min/1.73 m2 is apparent. The increased risk is attenuated after adjustment for other known risk factors, but remains statistically significant. Reproduced with permission.9
Recommendations for antihypertensive therapy in the general population are based on observational studies and controlled trials relating blood pressure level and antihypertensive therapy to CVD risk. Few patients with CKD were included in these studies. Thus, recommendations to reduce CVD risk in CKD are based largely on extrapolation from the general population.
Progression of CKD
Most kidney diseases worsen progressively over time. Antihypertensive therapy affects several modifiable key factors related to the progression of kidney disease, including hypertension, proteinuria, and other mechanisms, such as increased activity of the renin-angiotensin system (RAS) (Fig 7). Several large, controlled trials have examined the effect of antihypertensive therapy on the progression of kidney disease in patients with and without hypertension. While these trials have provided important answers about therapy, the relationships among these "progression factors" are complex, and many questions remain unanswered, especially regarding the mechanisms underlying the therapeutic benefit of the interventions.
Fig 7. Risk factors for kidney disease progression related to hypertension. Shaded ellipses represent stages of kidney disease (see Fig 2). Thick arrows between ellipses represent "risk factors" associated with progression of disease that can be affected by antihypertensive therapy. Thin arrows represent relationships between risk factors. Dashed lines indicate hypothesized causal relationships.
Based on these considerations, the Work Group defined the following goals for antihypertensive therapy in CKD (Table 5) and strategies and therapeutic targets to achieve them (Table 6). This formulation is consistent with the JNC 7 report, which recommends lifestyle modifications and pharmacological therapy to lower blood pressure and reduce CVD risk, with modifications for "compelling indications," including CKD.5
As indicated in Table 6, the Work Group recommended that clinicians consider reducing proteinuria as a goal for antihypertensive therapy in CKD. Proteinuria is important in CKD for a number of reasons (Table 7). There is strong evidence that proteinuria is a marker of kidney damage, and its presence identifies individuals with CKD. Large amounts of proteinuria are a clue to the type (diagnosis) of CKD. Higher levels of proteinuria are a risk factor for faster progression of CKD and development of CVD. Higher levels of proteinuria also identify individuals who benefit more from antihypertensive therapy. However, the Work Group decided that the evidence is not strong enough to conclude that proteinuria is a surrogate outcome for kidney disease progression. However, it was the opinion of the Work Group that proteinuria should be monitored during the course of CKD, and that under some circumstances, it would be appropriate to consider modifications to the antihypertensive regimen in patients with large amounts of proteinuria, such as a lower blood pressure goal or measures to reduce proteinuria. In general, these modifications should be undertaken in consultation with a nephrologist. The Work Group strongly recommended further research on this topic.
The Work Group was convened by the NKF Kidney Disease Outcomes Quality Initiative (K/DOQI) in response to recommendations of the NKF Task Force on CVD (Fig 8). The overall aim of the Work Group was to develop evidence-based recommendations for the evaluation and management of hypertension and use of antihypertensive agents in CKD. Topics considered are listed in Table 8. Based on the results of clinical and epidemiological studies, the Work Group defined the target population for these guidelines as patients with CKD Stages 1-4. Patients with CKD Stage 5 (kidney failure) were excluded since kidney disease progression may not be as important in patients who have already reached the stage of kidney failure, because the relationship between CVD risk and level of blood pressure is complex in kidney failure, and because of intermittent fluid shifts that affect blood pressure in hemodialysis patients. Thus, the Work Group concluded that the evidence base was not sufficient to develop strong recommendations for patients with kidney failure, and that extrapolation from the general population or from populations with earlier stages of CKD to those with kidney failure may not be appropriate. The Work Group acknowledges the importance of this topic, which will be addressed in a forthcoming K/DOQI Clinical Practice Guideline.
Fig 8. Evolution of National Kidney Foundation Guidelines on Hypertension and Antihypertensive Agents in CKD. Abbreviations: Cr, serum creatinine concentration; NKF, National Kidney Foundation; CVD, cardiovascular disease; GFR, glomerular filtration rate; CKD, chronic kidney disease; K/DOQI, Kidney Disease Outcomes Quality Initiative. To convert serum creatinine from mg/dL to mmol/L, multiply by 88.4.
The Work Group has included recommendations for both adults and children. Guideline 13 for children was written with careful consideration of past recommendations for the treatment of hypertension in children by JNC reports and by the National High Blood Pressure Education Program Work Group for Children and Adolescents.10
Two topics are highlighted in the Guidelines in which evidence is rapidly accumulating, but the evidence base is not yet sufficient for strong or moderately strong recommendations: use of ambulatory blood pressure monitoring (Guideline 3 and Appendix 3) and additional interventions for patients with large amounts of proteinuria (Background, Guidelines 1, 8, 9, 10, 11). For these topics, recommendations were based on weak evidence and opinion.
The Work Group developed the following operational definitions for these guidelines:
Antihypertensive therapy includes lifestyle modifications and pharmacological therapy that reduce blood pressure, in patients with or without hypertension.
Lifestyle modifications include changes in diet, exercise, and habits that may slow the progression of CKD or lower the risk of CVD. These guidelines focus specifically on lifestyle modifications that lower blood pressure, and are discussed in more detail in Guideline 6.
Pharmacological therapy includes selection of antihypertensive agents and blood pressure goals. General principles of pharmacological therapy and target blood pressure for CVD risk reduction are discussed in Guideline 7.
Antihypertensive agents are defined as agents that are usually prescribed to lower blood pressure. Many antihypertensive agents have effects in addition to lowering systemic blood pressure and are used for indications other than hypertension. Other agents may also lower blood pressure as a side-effect.
"Preferred agents" are classes of antihypertensive agents that have beneficial effects on progression of CKD or reducing CVD risk in addition to their antihypertensive effects, such as reducing proteinuria, slowing GFR decline, and inhibiting other pathogenetic mechanisms of kidney disease progression and CVD. In certain types of CKD, specific classes of antihypertensive agents, notably those that inhibit the renin-angiotensin system (RAS), are preferred agents for slowing progression of CKD. Thus, the Work Group recommended use of specific classes of antihypertensive agents in certain types of CKD, even if hypertension is not present. Preferred agents for specific types of CVD are discussed in Guideline 7. Preferred agents for CKD are discussed in Guidelines 8, 9, 10. ACE inhibitors and angiotensin receptor blockers are discussed in Guideline 11, and diuretics are discussed in Guideline 12.
These guidelines are intended for use by physicians, nurse practitioners, registered nurses, registered dietitians, masters prepared social workers, pharmacists, physician assistants, and other professionals who provide health care for patients with CKD. The information contained in these guidelines can and should be conveyed to patients and their families in an understandable manner by their physician and/or other health-care professionals. The development of educational support materials designed specifically for patients and their families should be part of the implementation of these guidelines.
All guidelines should be updated whenever new, pertinent information becomes available. To anticipate when these guidelines may need to be updated, the Work Group reviewed ongoing controlled trials in the general population and in patients with CKD (Appendix 1), as those results may be pertinent to some recommendations. Given the potential for these and other studies to provide information pertinent to the assessment and treatment of hypertension in patients with CKD, it was concluded that these guidelines should be updated in about 3 to 4 years from the time of publication, and sooner if new, pertinent information becomes available before then. The K/DOQI Work Group and the Advisory Board will monitor the progress of these trials and other developments in this area and recommend updating these guidelines as indicated.
K/DOQI Principles and Process
The development of these guidelines followed four basic principles set forth by K/DOQI (Table 9). The guidelines were developed using an evidence-based approach similar to that endorsed by the Agency for Health-Care Research and Quality.11 The Work Group reviewed all pertinent, published evidence in CKD, and critically appraised the quality of studies and the overall strength of evidence supporting each recommendation (Table 10). Additional details are given in Appendix 1 (Methods).
A systematic review of pharmacological blood pressure trials with CVD outcomes in the general population was felt to be beyond the scope of the Work Group. Since JNC 7 was not yet available at the time these guidelines were being developed, it was decided to review JNC 6 and guidelines issued since then for recommendations on blood pressure targets and specific antihypertensive agents. These guidelines are reviewed in Guideline 7.
This document contains 13 guidelines. The format for each guideline is outlined in Table 11. Each guideline contains one or more specific, numbered "recommendations" or "guideline statements." Each guideline contains background information, which is generally sufficient to interpret the guideline. The rationale for each guideline contains definitions, if appropriate, and a section on the strength of evidence. The strength of evidence includes a series of specific "rationale statements," each supported by evidence, and "summary tables" (if appropriate) compiling and evaluating original reports of studies. The guideline concludes with a discussion of limitations of the evidence review and a brief discussion of clinical applications, implementation issues and research recommendations regarding the topic.
Review of Evidence
Details of the search strategies and study selection are provided in Appendix A1. Table 12 lists the details of the literature search and review for each topic. Overall, 11,688 abstracts were screened by the Evidence Review Team, 899 articles were retrieved and reviewed, and data were extracted from 177 articles. Forty-seven articles were added by the Work Group. Finally, results from 76 articles were systematically listed in the summary tables in these guidelines.
The Work Group evaluated the quality of each study, the strength of evidence derived from the compilation of the studies, and the strength of each guideline recommendation based on the strength of evidence and other factors.
Summary tables succinctly describe the characteristics for each study according to five dimensions: study size, applicability (generalizability, based on the type of study subjects), baseline information, results based on the primary outcome (either progression of CKD or development of CVD), and methodological quality (Table 13). Only the primary outcome is represented, because studies were generally not powered to determine efficacy of interventions on secondary outcomes. Progression of kidney disease was defined by variables related to decline in GFR, such as doubling of serum creatinine or development of kidney failure (generally referred to as "end-stage renal disease" or ESRD), but often included mortality as part of a composite outcome. Development of CVD was defined by clinical events, cause-specific mortality, or total mortality. Surrogate outcomes for CKD and CVD included variables related to proteinuria or left ventricular hypertrophy, respectively. Within each of the summary tables, studies are ordered first by methodological quality (highest to lowest), then by applicability (most to least), and then by study size (largest to smallest).
The number of patients (N, sample size) is used as a measure of the weight of the evidence. In general, large studies provide more precise estimates of effects or associations. In addition, results from large studies are more likely generalizable; however, large size alone does not guarantee a high degree of applicability. A study that enrolls a large number of selected patients may be less generalizable than several smaller studies that include a broad spectrum of patient populations.
Applicability (also known as generalizability or external validity) addresses the issue of whether the study sample is sufficiently broad so that the results can be applied to the population of interest at large. The study sample is defined by the inclusion and exclusion criteria. A designation for applicability was assigned to each article, according to a three-level scale. In making this assessment, causes of CKD (diabetic kidney disease, nondiabetic kidney disease, and kidney disease in the kidney transplant) and sociodemographic characteristics were the primary determinants of applicability. If the study is not considered broadly generalizable, reasons for the limited applicability are reported. Table 14 describes the approach to assessing applicability.
In addition, baseline level of GFR (or serum creatinine), proteinuria, and blood pressure are reported to aid in the interpretation of applicability and results. For consistency, baseline data from the control group are reported.
The type of results available in each study is determined by the study design, the purpose, and the question(s) being asked. Therefore, the format of the results varies across summary tables. For comparisons of blood pressure targets or antihypertensive agents as shown in Table 15, only the pre-specified primary outcomes are reported, as the sample size may not have been sufficient to evaluate secondary outcomes. For studies reporting adverse effects of ACE inhibitors or ARBs, the percentages of adverse effects are reported.
Methodological quality (or internal validity) refers to the design, conduct, and reporting of the clinical study. Because studies with a variety of types of design were evaluated, a generic three-level classification of study quality was devised (Table 16).
In addition to original articles, we included review articles and selected original articles for topics that were determined, a priori, not to require a systematic review of the literature. Work Group members had wide latitude in summarizing these articles. The use of published or derived tables and figures was encouraged to simplify the presentation.
Extrapolation of Evidence From Studies in the General Population to Patients With CKD
Some guideline statements in this document are based on evidence derived from studies on surrogate outcomes (proteinuria or left ventricular hypertrophy) in the target population, as well as evidence derived from studies on clinical outcomes (kidney disease progression or clinical CVD outcomes) in the general population and extrapolated to the target population. Some would argue that no guideline statements should be made in the absence of evidence on clinical outcomes in the target population. However, the limited number of studies on antihypertensive therapy for CVD in CKD, compared to the large number of studies in the general population, required development of criteria for extrapolating evidence from the general population to the target population. The Work Group adopted the criteria developed by the NKF Task Force on Cardiovascular Disease in Chronic Renal Disease for extrapolating evidence for CVD outcomes or mortality from the general population to the target population3,4 (Table 17).
To extrapolate evidence from studies on treatment of hypertension with CVD outcomes, the following assumptions had to be met:
1. The mechanisms and expression of CVD in CKD should be similar to those observed in the general population. Specifically, the features of CVD, the relationship of the risk factor (hypertension) to CVD outcomes, the mechanism of risk factor alterations (blood pressure lowering), and the responsiveness of risk factors to therapies (lifestyle modifications and pharmacological therapy) should be similar in the general populations and in patients with CKD.
2. Therapies in patients with CKD should be as safe, or nearly so, as in the general population. In particular, there should not be additional adverse effects of a specific therapy that limits its usefulness in patients with CKD, either because of altered pharmacokinetics, drug interactions, or increased risk of toxicity to the kidney.
3. The duration of therapy required to improve CVD outcomes in the general population should not exceed the life expectancy of patients with CKD. In other words, it should not already be too late to intervene in this generally elderly, sick, and frail population. Determining whether patients with CKD can survive for long enough to gain the benefit of therapy for CVD is a difficult question. Numerous studies show a dramatically shortened life expectancy for patients with CKD, especially patients with kidney failure. For example, the USRDS has estimated that the average life expectancy of 60- to 64-year-old patients treated by dialysis ranges from 3.6 to 5.1 years, depending on gender and race. On the other hand, the most common cause of death in kidney failure is CVD, and numerous studies of CVD in the general population have shown a benefit of interventions within 2 to 5 years, with greater and earlier benefits in patients at highest risk. Thus, it is likely that patient with kidney failure could benefit from more effective treatment of CVD. Because of their longer life expectancy, patients with earlier stages of CKD might be most likely to benefit.
Rating Strength of Evidence and Strength of Recommendations
The strength of evidence for a group of studies (for example, the studies in an evidence table) was graded using a rating system that takes into account (1) methodological quality of the studies; (2) target population (patients with CKD or other populations); and (3) study outcomes (health outcomes or surrogate measures for those outcomes) (Table 18).13
The overall strength of each recommendation (guideline statement) was then rated (Table 19), principally according to the strength of evidence, but also according to other factors, such as practicality.13,14 Cost was not systematically taken into account.
In addition, the Work Group adopted an approach for using existing expert guidelines issued for populations other than the target population. Grades assigned by the guideline-issuing bodies for the strength of evidence were adopted. When the strength of the recommendation or the strength of evidence was not graded explicitly, this Work Group assumed that the guideline would be based on at least moderately strong evidence. The extrapolation of these guideline recommendations from the general populations to the target population was considered to support grade B recommendations.
Our approach has limitations. While the literature searches were intended to be comprehensive, they were not exhaustive. Medline was the only database searched, and searches were limited to English language publications. Hand searches of journals were not performed, and review articles and textbook chapters were not systematically searched. Important studies known to the domain experts that were missed by the literature search were included in the review. Additional essential studies identified during the review process were individually considered for inclusion.
Table 20 summarizes key messages and recommendations of the Work Group. Table 21 summarizes the major recommendations for target blood pressure, preferred antihypertensive agents to slow progression of kidney disease, and additional agents to reduce CVD risk and achieve target blood pressure, for types of CKD.
An algorithm to illustrate decision-making in blood pressure management in CKD is shown in Fig 9.
Table 22 summarizes recommendations regarding proteinuria, irrespective of the type of kidney disease.
Fig 9. Algorithm for evaluation and management of hypertension and use of antihypertensive agents in CKD.
Guideline 1: Goals of Antihypertensive Therapy in CKD
The numbering of tables in this section corresponds to numbering in later text, and is not consecutive.
Hypertension is common in CKD and is a risk factor for faster progression of kidney disease and development and worsening of cardiovascular disease (CVD). Some antihypertensive agents also slow the progression of kidney disease by mechanisms in addition to their antihypertensive effect.
1.1 Antihypertensive therapy should be used in CKD to:
1.1.a Lower blood pressure (A);
1.1.b Reduce the risk of CVD, in patients with or without hypertension (B) (see Guideline 7);
1.1.c Slow progression of kidney disease, in patients with or without hypertension (A) (see Guidelines 8, 9, 10).
1.2 Modifications to antihypertensive therapy should be considered based on the level of proteinuria during treatment (C) (see Guidelines 8, 9, 10, 11).
1.3 Antihypertensive therapy should be coordinated with other therapies for CKD as part of a multi-intervention strategy (A).
1.4 If there is a discrepancy between the treatment recommended to slow progression of CKD and to reduce the risk of CVD, individual decision-making should be based on risk stratification (C).
Guideline 2: Evaluation of Patients With Chronic Kidney Disease or Hypertension
Careful initial evaluation and frequent re-evaluation are essential for effective treatment of hypertension and use of antihypertensive agents in CKD. Because CKD and hypertension are often present together and both are generally asymptomatic, Guideline 2 considers evaluations of patients with either condition.
2.1 Blood pressure should be measured at each health encounter (A).
2.2 Initial evaluation should include the following elements:
2.2.a Description of CKD;
2.2.a.i Type (diagnosis), level of GFR, and level of proteinuria (Table 49) (A);
2.2.a.ii Complications of decreased GFR (A);
2.2.a.iii Risk for progression of kidney disease (A).
2.2.b Presence of clinical CVD and CVD risk factors (Table 50) (A);
2.2.c Comorbid conditions (A);
2.2.d Barriers to self-management, adherence to diet and other lifestyle modifications, adherence to pharmacological therapy (see Guidelines 5, 6, and 7) (B);
2.2.e Complications of pharmacological therapy (see Guidelines 7, 11-12) (A).
2.3 A clinical action plan should be developed for each patient, based on the stage of CKD (Table 51) (B).
2.4 Recommended intervals for follow-up evaluation should be guided by clinical conditions (Table 52) (C).
2.5 Patients with resistant hypertension should undergo additional evaluation to ascertain the cause (B).
2.6 Patients should be referred to specialists, when possible, for the following indications (Table 53).
Guideline 3: Measurement of Blood Pressure in Adults
Blood pressure can be determined by resting blood pressure measurement in the health-care provider’s office (casual blood pressure [CBP]), self-measured blood pressure (SMBP), or ambulatory blood pressure monitoring (ABPM).
3.1 Blood pressure should be measured according to the recommendations for indirect measurement of arterial blood pressure of the American Heart Association and Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) (A).
3.2 Patients should be taught to measure and record their blood pressure, whenever possible (C).
3.3 Ambulatory blood pressure monitoring should be considered for patients with CKD for the following indications (C):
3.3.a Suspected white coat hypertension
3.3.b Resistant hypertension
3.3.c Hypotensive symptoms while taking antihypertensive medications
3.3.d Episodic hypertension
3.3.e Autonomic dysfunction
Guideline 4: Evaluation for Renal Artery Disease
Renal artery disease (RAD) is a cause of CKD and hypertension and can be present in patients with other causes of CKD, such as diabetes or hypertensive nephrosclerosis, and CKD in the kidney transplant.
4.1 For patients in whom there is a clinical suspicion of RAD, the clinician should do one or more of the following:
4.1.a Estimate the probability of RAD using a predictive index derived from clinical characteristics (B)
4.1.b Obtain a noninvasive screening test for RAD (A)
4.1.c Refer to a kidney disease or hypertension specialist for evaluation (C).
4.2 Patients found to have hemodynamically significant RAD should be referred to a kidney disease or hypertension specialist for management (C).
Guideline 5: Education on Self-Management Behavior
Antihypertensive therapy must take into consideration the patient’s perception of the health-care provider’s advice and prescriptions, factors that may influence self-management behaviors, and the likelihood that the patient will adhere to recommendations.
5.1 Self-management principles should be incorporated into the treatment plan (B).
5.2 Patient and family education about antihypertensive therapy should be culturally sensitive, sensitive to economic considerations, and based on the patient’s level of understanding (B).
5.3 All patients should be assessed for barriers to adherence and self-management (B), and referred for further counseling as needed to a nurse practitioner, registered nurse, registered dietitian, masters prepared social worker, pharmacist, physician assistant, or other professional (C).
Guideline 6: Dietary and Other Therapeutic Lifestyle Changes in Adults
Dietary and other therapeutic lifestyle modifications are recommended as part of a comprehensive strategy to lower blood pressure and reduce CVD risk in CKD.
6.1 Dietary sodium intake of less than 2.4 g/d (less than 100 mmol/d) should be recommended in most adults with CKD and hypertension (A).
6.2 Other dietary recommendations for adults should be modified according to the stage of CKD (Table 83) (B).
6.3 Lifestyle modifications recommended for CVD risk reduction should be recommended as part of the treatment regimen (Table 84) (B).
6.4 Referral to a registered dietitian should be considered to help patients achieve dietary recommendations (C).
Guideline 7: Pharmacological Therapy: Use of Antihypertensive Agents in CKD
All antihypertensive agents can be used to lower blood pressure in CKD. Multi-drug regimens will be necessary in most patients with CKD to achieve therapeutic goals. Patients with specific causes of kidney disease and CVD will benefit from specific classes of agents.
7.1 Patients with CKD should be considered in the "highest-risk" group for CVD for implementing recommendations for pharmacological therapy, irrespective of cause of CKD (A).
7.2 Target blood pressure for CVD risk reduction in CKD should be <130/80 mm Hg (B).
7.3 Antihypertensive agents should be prescribed as follows, when possible:
7.3.a Preferred agents for CKD should be used first (see Guidelines 8, 9, 10, 11) (A);
7.3.b Diuretics should be included in the antihypertensive regimen in most patients (A).
7.3.c Choose additional agents based on cardiovascular disease-specific indications to achieve therapeutic and preventive targets (Table 86) and to avoid side-effects and interactions (B).
7.4 The antihypertensive regimen should be simplified as much as possible (B).
7.4.a Long-acting (once-daily agents) should be used when possible (B).
7.4.b Two agents, either as separate prescriptions or as a fixed-dose combination containing preferred agents, may be considered as initial therapy for SBP >20 mm Hg above goal according to the stage of CKD and CVD risk (C).
7.4.c Fixed-dose combinations may be used for maintenance therapy after the antihypertensive regimen has been established (B).
Guideline 8: Pharmacological Therapy: Diabetic Kidney Disease
Diabetes mellitus is the most common cause of kidney failure in the United States. Diabetic kidney disease is characterized by the early onset of albuminuria, hypertension, and a high risk of coexistent or subsequent CVD.
8.1 Target blood pressure in diabetic kidney disease should be <130/80 mm Hg (Guideline 7) (Table 104).
8.2 Patients with diabetic kidney disease, with or without hypertension, should be treated with an ACE inhibitor or an ARB (Table 104).
Guideline 9: Pharmacological Therapy: Nondiabetic Kidney Disease
Nondiabetic kidney diseases include glomerular diseases other than diabetes, vascular diseases other than renal artery disease, tubulointerstitial diseases, and cystic disease. Among these diseases, the level of proteinuria is useful for diagnosis and prognosis. Glomerular diseases are characterized by higher levels of proteinuria than other diseases. Higher levels of proteinuria are associated with faster progression of kidney disease and increased risk of CVD.
9.1 Target blood pressure in nondiabetic kidney disease should be <130/80 mm Hg (Guideline 1) (Table 111).
9.2 Patients with nondiabetic kidney disease and spot urine total protein to creatinine ratio ≥ 200 mg/g, with or without hypertension, should be treated with an ACE inhibitor or ARB (Table 111).
Guideline 10: Pharmacological Therapy: Kidney Disease in the Kidney Transplant Recipient
Most kidney transplant recipients have CKD and hypertension. High blood pressure in kidney transplant recipients is a risk factor for faster progression of CKD and development of CVD.
10.1 The target blood pressure in kidney transplant recipients should be <130/80 mm Hg (see Guideline 7) (Table 119).
10.2 Patients with CKD in the kidney transplant should be treated with any of the following to reach the target blood pressure: CCB, diuretics, ACE inhibitor, ARB, or beta-blocker (Table 119).
Guideline 11: Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in CKD
ACE inhibitors and ARBs can be used safely in most patients with CKD.
11.1 ACE inhibitors and ARBs should be used at moderate to high doses, as used in clinical trials) (A).
11.2 ACE inhibitors and ARBs should be used as alternatives to each other, if the preferred class cannot be used (B).
11.3 ACE inhibitors and ARBs can be used in combination to lower blood pressure or reduce proteinuria (C).
11.4 Patients treated with ACE inhibitors or ARBs should be monitored for hypotension, decreased GFR, and hyperkalemia (A).
11.5 The interval for monitoring blood pressure, GFR, and serum potassium depends on baseline levels (Table 123) (B).
11.6 In most patients, the ACE inhibitor or ARB can be continued if:
11.6.a GFR decline over four months is <30% from baseline value (B);
11.6.b Serum potassium is <5.5 mEq/L (B).
11.7 ACE inhibitors and ARBs should not be used or used with caution in certain circumstances (Table 124).
Guideline 12: Use of Diuretics in CKD
Diuretics are useful in the management of most patients with CKD. They reduce ECF volume, lower blood pressure, potentiate the effects of ACE inhibitors, ARBs, and other antihypertensive agents and reduce the risk of CVD in CKD. Choice of diuretic agents depends on the level of GFR and need for reduction in ECF volume.
12.1 Most patients with CKD should be treated with a diuretic (A).
12.1.a Thiazide diuretics given once daily are recommended in patients with GFR ≥ 30 mL/min/1.73 m2 (CKD Stages 1-3) (A);
12.1.b Loop diuretics given once or twice daily are recommended in patients with GFR <30 mL/min/1.73 m2 (CKD Stages 4-5) (A);
12.1.c Loop diuretics given once or twice daily, in combination with thiazide diuretics, can be used for patients with ECF volume expansion and edema (A).
12.1.d Potassium-sparing diuretics should be used with caution:
12.1.d.i In patients with GFR <30 mL/min/1.73 m2 (CKD Stages 4-5) (A);
12.1.d.ii In patients receiving concomitant therapy with ACE inhibitors or ARBs (A);
12.1.d.iii In patients with additional risk factors for hyperkalemia (A).
12.2 Patients treated with diuretics should be monitored for:
12.2.a Volume depletion, manifest by hypotension or decreased GFR (A);
12.2.b Hypokalemia and other electrolyte abnormalities (A).
12.2.c The interval for monitoring depends on baseline values for blood pressure, GFR, and serum potassium concentration (Table 145) (B).
12.3 Long-acting diuretics and combinations of diuretics with other antihypertensive agents should be considered to increase patient adherence (B).
Guideline 13: Special Considerations in Children
Hypertension is common in children with CKD. Because of their young age at onset of CKD and hypertension, children have a high lifetime exposure to risk factors for CVD. Thus, children with CKD are at high risk of complications from hypertension.
13.1 Measurement of blood pressure in children should be performed with age- and size-appropriate equipment, and blood pressure values should be interpreted according to normal values adjusted for age, gender, and height percentile, as recommended by the 1996 Update on the Task Force Report on High Blood Pressure in Children and Adolescents: A Working Group Report from the National High Blood Pressure Education Program (A).
13.2 The cause of CKD and age of the child should be considered in selecting the class of antihypertensive agent (A).
13.3 Target blood pressure in children should be lower than the 90th percentile for normal values adjusted for age, gender, and height or 130/80 mm Hg, whichever is lower (B).
13.4 Because of the specialized nature of CKD and blood pressure management in children, a pediatric kidney disease specialist should be involved in their care, when possible (C).